 Found 117952 hits with Last Name = 'li' and Initial = 'c'
Found 117952 hits with Last Name = 'li' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50122692

(CHEMBL282336 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1onc(C)c1C)-c1ncco1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C31H27F3N4O5S/c1-19-20(2)36-43-29(19)37-44(40,41)27-7-5-4-6-26(27)25-13-10-22(30-35-14-15-42-30)17-23(25)18-38(3)28(39)16-21-8-11-24(12-9-21)31(32,33)34/h4-15,17,37H,16,18H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelin A receptor expressed in CHO cells |
J Med Chem 46: 125-37 (2002)
Article DOI: 10.1021/jm020289q
BindingDB Entry DOI: 10.7270/Q2348M3R |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147824
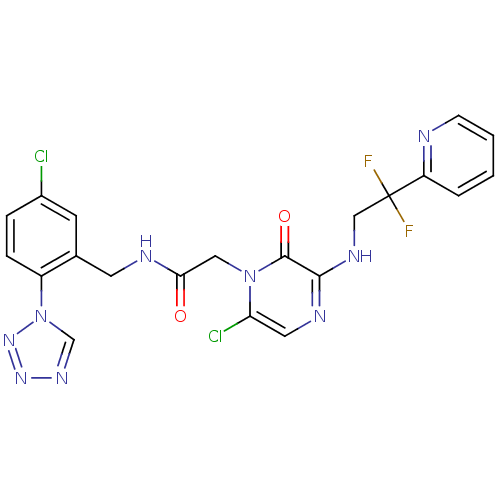
(2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H17Cl2F2N9O2/c22-14-4-5-15(34-12-30-31-32-34)13(7-14)8-27-18(35)10-33-17(23)9-28-19(20(33)36)29-11-21(24,25)16-3-1-2-6-26-16/h1-7,9,12H,8,10-11H2,(H,27,35)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147818
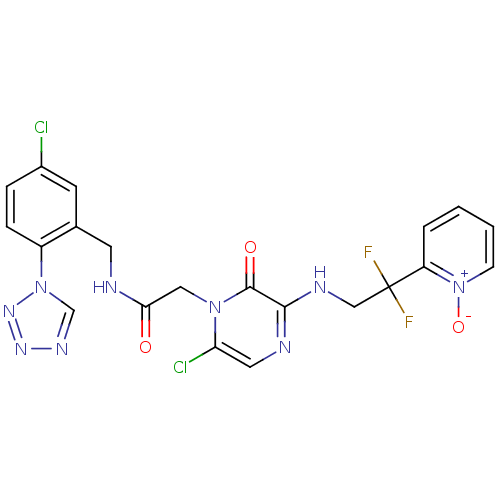
((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H17Cl2F2N9O3/c22-14-4-5-15(33-12-29-30-31-33)13(7-14)8-26-18(35)10-32-17(23)9-27-19(20(32)36)28-11-21(24,25)16-3-1-2-6-34(16)37/h1-7,9,12H,8,10-11H2,(H,26,35)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50147818

((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H17Cl2F2N9O3/c22-14-4-5-15(33-12-29-30-31-33)13(7-14)8-26-18(35)10-32-17(23)9-27-19(20(32)36)28-11-21(24,25)16-3-1-2-6-34(16)37/h1-7,9,12H,8,10-11H2,(H,26,35)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50147824

(2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H17Cl2F2N9O2/c22-14-4-5-15(34-12-30-31-32-34)13(7-14)8-27-18(35)10-33-17(23)9-28-19(20(33)36)29-11-21(24,25)16-3-1-2-6-26-16/h1-7,9,12H,8,10-11H2,(H,27,35)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50122706
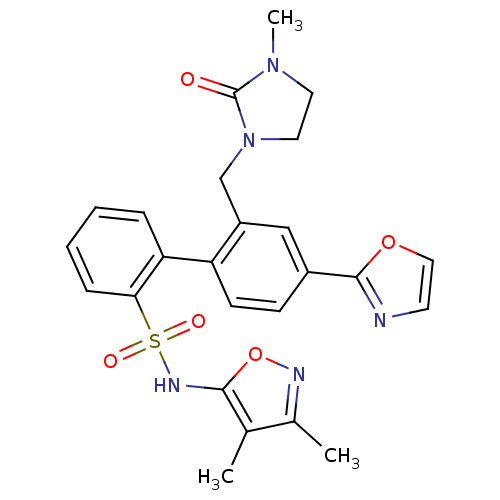
(2'-(3-Methyl-2-oxo-imidazolidin-1-ylmethyl)-4'-oxa...)Show SMILES CN1CCN(Cc2cc(ccc2-c2ccccc2S(=O)(=O)Nc2onc(C)c2C)-c2ncco2)C1=O Show InChI InChI=1S/C25H25N5O5S/c1-16-17(2)27-35-23(16)28-36(32,33)22-7-5-4-6-21(22)20-9-8-18(24-26-10-13-34-24)14-19(20)15-30-12-11-29(3)25(30)31/h4-10,13-14,28H,11-12,15H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelin A receptor expressed in CHO cells |
J Med Chem 46: 125-37 (2002)
Article DOI: 10.1021/jm020289q
BindingDB Entry DOI: 10.7270/Q2348M3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50122693
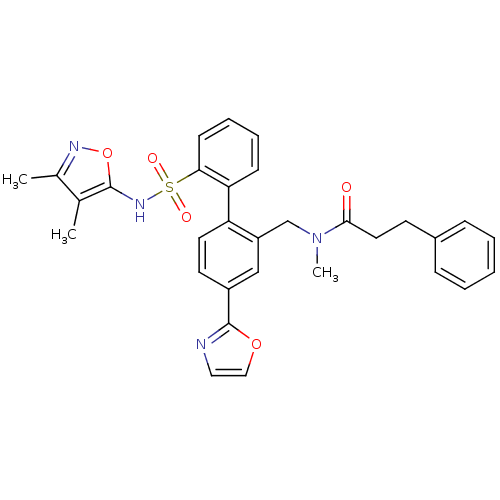
(CHEMBL29346 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsul...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1onc(C)c1C)-c1ncco1)C(=O)CCc1ccccc1 Show InChI InChI=1S/C31H30N4O5S/c1-21-22(2)33-40-30(21)34-41(37,38)28-12-8-7-11-27(28)26-15-14-24(31-32-17-18-39-31)19-25(26)20-35(3)29(36)16-13-23-9-5-4-6-10-23/h4-12,14-15,17-19,34H,13,16,20H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelin A receptor expressed in CHO cells |
J Med Chem 46: 125-37 (2002)
Article DOI: 10.1021/jm020289q
BindingDB Entry DOI: 10.7270/Q2348M3R |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50229232
(CHEMBL610527)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC=C2c1ccccc1)ccc3O |r,c:24,THB:10:9:17:4.5.6| Show InChI InChI=1S/C26H27NO3/c28-20-9-8-18-14-21-26(29)11-10-19(17-4-2-1-3-5-17)24-25(26,22(18)23(20)30-24)12-13-27(21)15-16-6-7-16/h1-5,8-10,16,21,24,28-29H,6-7,11-15H2/t21-,24+,25+,26-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Opioid receptor mu using [3H]DAMGO as radioligand in guinea pig brain membrane |
J Med Chem 34: 1715-20 (1991)
BindingDB Entry DOI: 10.7270/Q2T43TPG |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50122686
(1-Methyl-1H-indole-2-carboxylic acid [2'-(3,4-dime...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1onc(C)c1C)-c1ncco1)C(=O)c1cc2ccccc2n1C Show InChI InChI=1S/C32H29N5O5S/c1-20-21(2)34-42-30(20)35-43(39,40)29-12-8-6-10-26(29)25-14-13-23(31-33-15-16-41-31)17-24(25)19-36(3)32(38)28-18-22-9-5-7-11-27(22)37(28)4/h5-18,35H,19H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelin A receptor expressed in CHO cells |
J Med Chem 46: 125-37 (2002)
Article DOI: 10.1021/jm020289q
BindingDB Entry DOI: 10.7270/Q2348M3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50122694
(2'-(3,3-Dimethyl-2-oxo-pyrrolidin-1-ylmethyl)-4'-o...)Show SMILES Cc1noc(NS(=O)(=O)c2ccccc2-c2ccc(cc2CN2CCC(C)(C)C2=O)-c2ncco2)c1C Show InChI InChI=1S/C27H28N4O5S/c1-17-18(2)29-36-24(17)30-37(33,34)23-8-6-5-7-22(23)21-10-9-19(25-28-12-14-35-25)15-20(21)16-31-13-11-27(3,4)26(31)32/h5-10,12,14-15,30H,11,13,16H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelin A receptor expressed in CHO cells |
J Med Chem 46: 125-37 (2002)
Article DOI: 10.1021/jm020289q
BindingDB Entry DOI: 10.7270/Q2348M3R |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537072

(CHEMBL440072)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N Show InChI InChI=1S/C63H88N16O16S2/c1-34(66)53(84)69-30-51(83)70-48-32-96-97-33-49(63(94)95)78-60(91)47(31-80)77-62(93)52(35(2)81)79-55(86)42(22-12-14-24-65)71-58(89)45(27-38-29-68-40-20-10-9-19-39(38)40)75-57(88)44(26-37-17-7-4-8-18-37)73-56(87)43(25-36-15-5-3-6-16-36)74-59(90)46(28-50(67)82)76-54(85)41(72-61(48)92)21-11-13-23-64/h3-10,15-20,29,34-35,41-49,52,68,80-81H,11-14,21-28,30-33,64-66H2,1-2H3,(H2,67,82)(H,69,84)(H,70,83)(H,71,89)(H,72,92)(H,73,87)(H,74,90)(H,75,88)(H,76,85)(H,77,93)(H,78,91)(H,79,86)(H,94,95)/t34-,35+,41+,42-,43+,44-,45+,46-,47-,48+,49-,52+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50124947
(CHEMBL453539)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@]1([H])CSSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](CC(O)=O)NC2=O)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N2CCC[C@@]2([H])C(=O)N2CCC[C@@]2([H])C(=O)N[C@@]([H])([C@@H](C)CC)C(=O)N1 |r| Show InChI InChI=1S/C67H104N18O18S2/c1-6-35(3)51-62(99)79-44-33-104-105-34-45(78-55(92)39(20-13-25-71-67(69)70)73-49(88)31-72-54(91)41(30-50(89)90)75-60(97)46-21-14-26-83(46)64(101)42(76-58(44)95)29-38-17-9-8-10-18-38)59(96)82-53(37(5)87)63(100)74-40(19-11-12-24-68)56(93)77-43(32-86)57(94)81-52(36(4)7-2)66(103)85-28-16-23-48(85)65(102)84-27-15-22-47(84)61(98)80-51/h8-10,17-18,35-37,39-48,51-53,86-87H,6-7,11-16,19-34,68H2,1-5H3,(H,72,91)(H,73,88)(H,74,100)(H,75,97)(H,76,95)(H,77,93)(H,78,92)(H,79,99)(H,80,98)(H,81,94)(H,82,96)(H,89,90)(H4,69,70,71)/t35-,36-,37+,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,51-,52-,53-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of bovine beta-trypsin using Boc-QAR-MCA as substrate measured every 30 secs for 10 mins |
Eur J Med Chem 155: 695-704 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.029
BindingDB Entry DOI: 10.7270/Q29026CK |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50093262
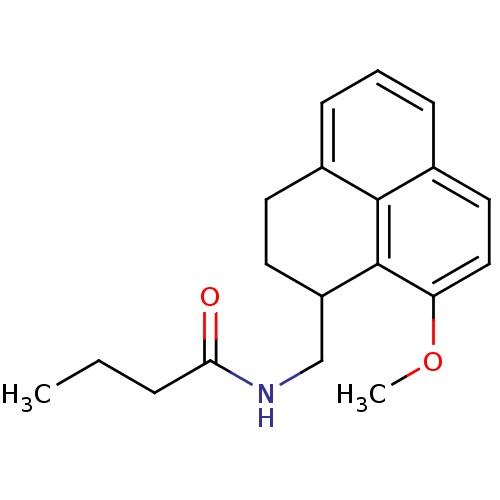
(CHEMBL131252 | N-(9-Methoxy-2,3-dihydro-1H-phenale...)Show InChI InChI=1S/C19H23NO2/c1-3-5-17(21)20-12-15-9-8-13-6-4-7-14-10-11-16(22-2)19(15)18(13)14/h4,6-7,10-11,15H,3,5,8-9,12H2,1-2H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Binding affinity for human melatonin receptor type 1A, expressed in HEK-293 cells (2-[125I]-Iodomelatonin is used as radioligand) |
J Med Chem 43: 4051-62 (2000)
BindingDB Entry DOI: 10.7270/Q20Z72JX |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM209097
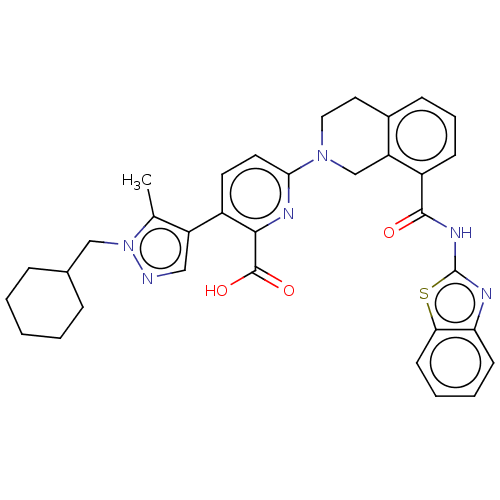
(US9266877, 43)Show SMILES Cc1c(cnn1CC1CCCCC1)-c1ccc(nc1C(O)=O)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C34H34N6O3S/c1-21-26(18-35-40(21)19-22-8-3-2-4-9-22)24-14-15-30(37-31(24)33(42)43)39-17-16-23-10-7-11-25(27(23)20-39)32(41)38-34-36-28-12-5-6-13-29(28)44-34/h5-7,10-15,18,22H,2-4,8-9,16-17,19-20H2,1H3,(H,42,43)(H,36,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50122715
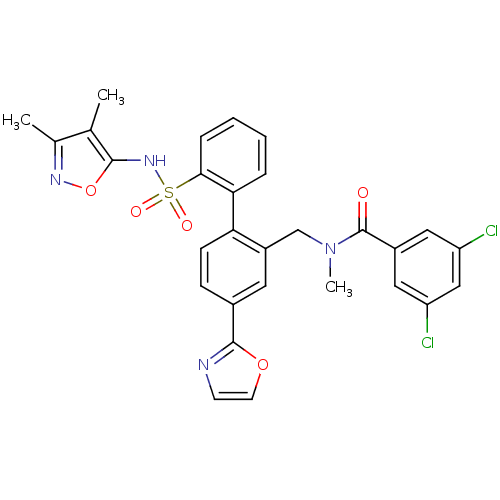
(3,5-Dichloro-N-[2'-(3,4-dimethyl-isoxazol-5-ylsulf...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1onc(C)c1C)-c1ncco1)C(=O)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C29H24Cl2N4O5S/c1-17-18(2)33-40-27(17)34-41(37,38)26-7-5-4-6-25(26)24-9-8-19(28-32-10-11-39-28)12-21(24)16-35(3)29(36)20-13-22(30)15-23(31)14-20/h4-15,34H,16H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelin A receptor expressed in CHO cells |
J Med Chem 46: 125-37 (2002)
Article DOI: 10.1021/jm020289q
BindingDB Entry DOI: 10.7270/Q2348M3R |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50096906
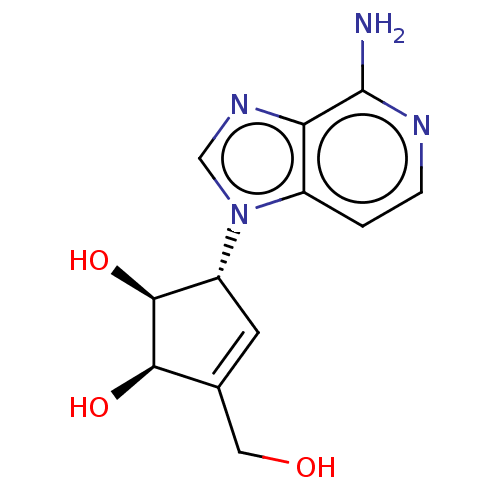
(CHEMBL154745 | US10227373, Compound D-3-Deazaisone...)Show SMILES Nc1nccc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |t:13| Show InChI InChI=1S/C12H14N4O3/c13-12-9-7(1-2-14-12)16(5-15-9)8-3-6(4-17)10(18)11(8)19/h1-3,5,8,10-11,17-19H,4H2,(H2,13,14)/t8-,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50096906

(CHEMBL154745 | US10227373, Compound D-3-Deazaisone...)Show SMILES Nc1nccc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |t:13| Show InChI InChI=1S/C12H14N4O3/c13-12-9-7(1-2-14-12)16(5-15-9)8-3-6(4-17)10(18)11(8)19/h1-3,5,8,10-11,17-19H,4H2,(H2,13,14)/t8-,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032
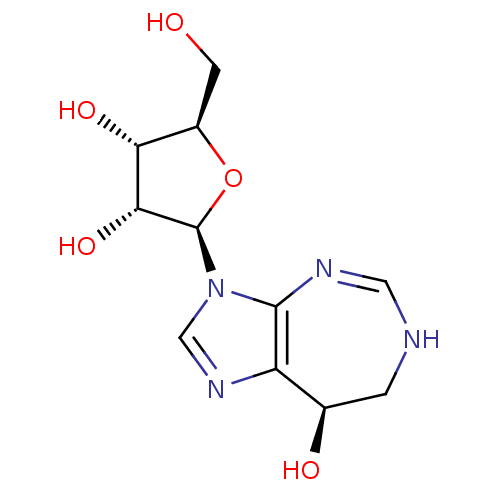
(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113877
BindingDB Entry DOI: 10.7270/Q2PK0M78 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50561528
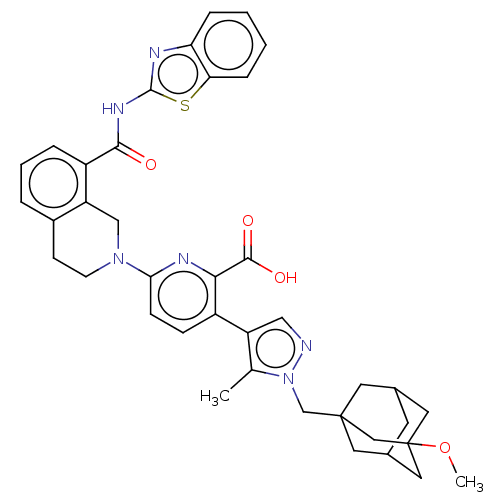
(CHEMBL4762875)Show SMILES COC12CC3CC(CC(Cn4ncc(c4C)-c4ccc(nc4C(O)=O)N4CCc5cccc(C(=O)Nc6nc7ccccc7s6)c5C4)(C3)C1)C2 |TLB:1:2:5.4.47:7,5:6:4.3.47:48,THB:5:4:6.49.7:48,3:4:7:49.2.48,3:2:5.4.47:7| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50162797
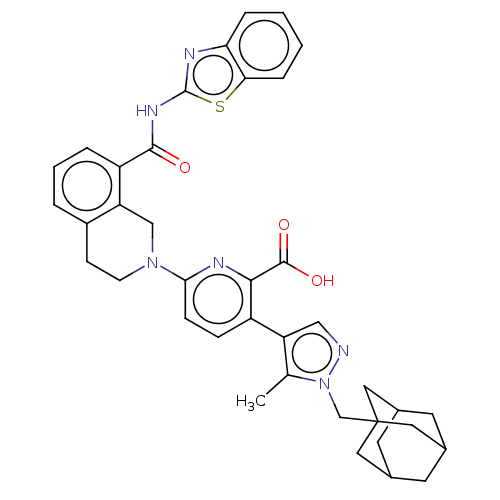
(CHEMBL3793424)Show SMILES Cc1c(cnn1CC12CC3CC(CC(C3)C1)C2)-c1ccc(nc1C(O)=O)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 |TLB:14:9:16:12.13.15,14:13:16:10.9.8,THB:12:11:8:14.13.15,12:13:10.11.16:8| Show InChI InChI=1S/C38H38N6O3S/c1-22-29(19-39-44(22)21-38-16-23-13-24(17-38)15-25(14-23)18-38)27-9-10-33(41-34(27)36(46)47)43-12-11-26-5-4-6-28(30(26)20-43)35(45)42-37-40-31-7-2-3-8-32(31)48-37/h2-10,19,23-25H,11-18,20-21H2,1H3,(H,46,47)(H,40,42,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573166
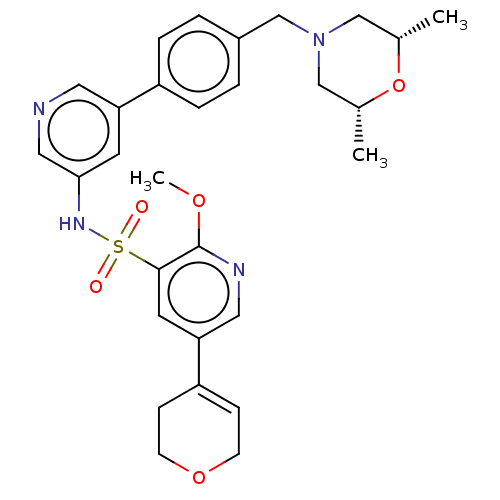
(CHEMBL4869783)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2C[C@H](C)O[C@H](C)C2)cc1)C1=CCOCC1 |r,t:37| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50573157
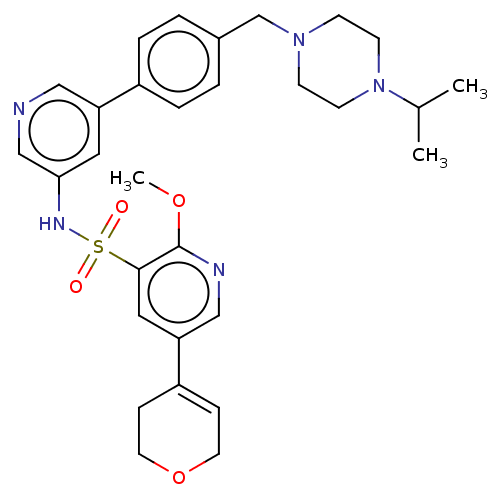
(CHEMBL4850297)Show SMILES COc1ncc(cc1S(=O)(=O)Nc1cncc(c1)-c1ccc(CN2CCN(CC2)C(C)C)cc1)C1=CCOCC1 |t:38| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length P110delta/full length untagged human p85alpha expressed in baculovirus infected Sf... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01102
BindingDB Entry DOI: 10.7270/Q2N87FKX |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50457933

(CHEMBL327265)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O Show InChI InChI=1S/C22H18Cl2F2N8O3/c23-15-4-5-16(33-13-27-12-31-33)14(7-15)8-28-19(35)10-32-18(24)9-29-20(21(32)36)30-11-22(25,26)17-3-1-2-6-34(17)37/h1-7,9,12-13H,8,10-11H2,(H,28,35)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50457929
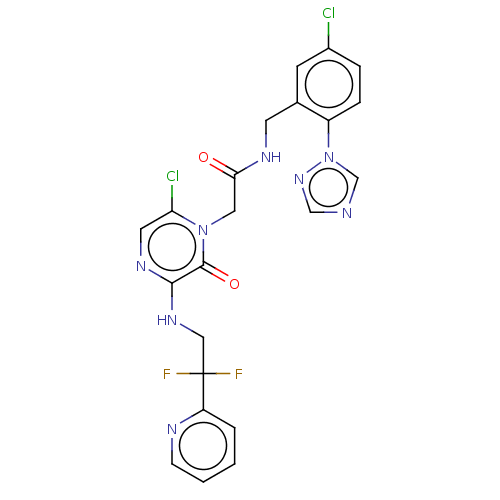
(CHEMBL104951)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O)c1ccccn1 Show InChI InChI=1S/C22H18Cl2F2N8O2/c23-15-4-5-16(34-13-27-12-32-34)14(7-15)8-29-19(35)10-33-18(24)9-30-20(21(33)36)31-11-22(25,26)17-3-1-2-6-28-17/h1-7,9,12-13H,8,10-11H2,(H,29,35)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50457933

(CHEMBL327265)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O Show InChI InChI=1S/C22H18Cl2F2N8O3/c23-15-4-5-16(33-13-27-12-31-33)14(7-15)8-28-19(35)10-32-18(24)9-29-20(21(32)36)30-11-22(25,26)17-3-1-2-6-34(17)37/h1-7,9,12-13H,8,10-11H2,(H,28,35)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50457929

(CHEMBL104951)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O)c1ccccn1 Show InChI InChI=1S/C22H18Cl2F2N8O2/c23-15-4-5-16(34-13-27-12-32-34)14(7-15)8-29-19(35)10-33-18(24)9-30-20(21(33)36)31-11-22(25,26)17-3-1-2-6-28-17/h1-7,9,12-13H,8,10-11H2,(H,29,35)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464108
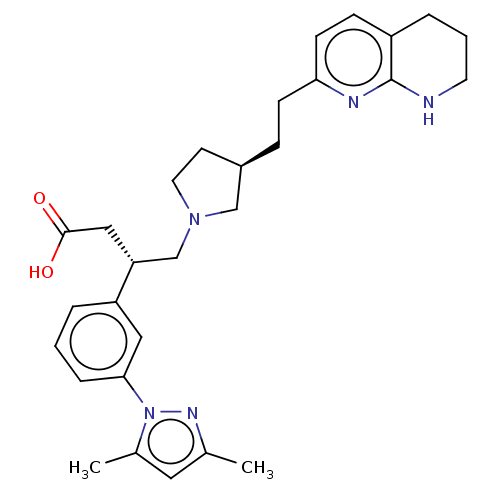
(CHEMBL4241824)Show SMILES Cc1cc(C)n(n1)-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C29H37N5O2/c1-20-15-21(2)34(32-20)27-7-3-5-24(16-27)25(17-28(35)36)19-33-14-12-22(18-33)8-10-26-11-9-23-6-4-13-30-29(23)31-26/h3,5,7,9,11,15-16,22,25H,4,6,8,10,12-14,17-19H2,1-2H3,(H,30,31)(H,35,36)/t22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to human integrin alphaVbeta6 assessed as dissociation constant up to 48 hrs by liquid scintillation counting |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50122676
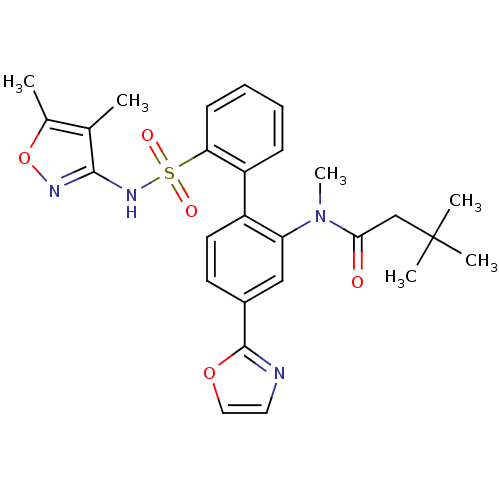
(CHEMBL274489 | N-[2'-(4,5-Dimethyl-isoxazol-3-ylsu...)Show SMILES CN(C(=O)CC(C)(C)C)c1cc(ccc1-c1ccccc1S(=O)(=O)Nc1noc(C)c1C)-c1ncco1 Show InChI InChI=1S/C27H30N4O5S/c1-17-18(2)36-29-25(17)30-37(33,34)23-10-8-7-9-21(23)20-12-11-19(26-28-13-14-35-26)15-22(20)31(6)24(32)16-27(3,4)5/h7-15H,16H2,1-6H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelin A receptor expressed in CHO cells |
J Med Chem 46: 125-37 (2002)
Article DOI: 10.1021/jm020289q
BindingDB Entry DOI: 10.7270/Q2348M3R |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50122712

(CHEMBL440780 | N-[2'-(3,4-Dimethyl-isoxazol-5-ylsu...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1onc(C)c1C)-c1ncco1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C30H28N4O5S/c1-20-21(2)32-39-29(20)33-40(36,37)27-12-8-7-11-26(27)25-14-13-23(30-31-15-16-38-30)18-24(25)19-34(3)28(35)17-22-9-5-4-6-10-22/h4-16,18,33H,17,19H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelin A receptor expressed in CHO cells |
J Med Chem 46: 125-37 (2002)
Article DOI: 10.1021/jm020289q
BindingDB Entry DOI: 10.7270/Q2348M3R |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50229232

(CHEMBL610527)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)CC=C2c1ccccc1)ccc3O |r,c:24,THB:10:9:17:4.5.6| Show InChI InChI=1S/C26H27NO3/c28-20-9-8-18-14-21-26(29)11-10-19(17-4-2-1-3-5-17)24-25(26,22(18)23(20)30-24)12-13-27(21)15-16-6-7-16/h1-5,8-10,16,21,24,28-29H,6-7,11-15H2/t21-,24+,25+,26-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to opioid receptor kappa using [3H]-EK as radioligand in guinea pig brain membrane |
J Med Chem 34: 1715-20 (1991)
BindingDB Entry DOI: 10.7270/Q2T43TPG |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030759
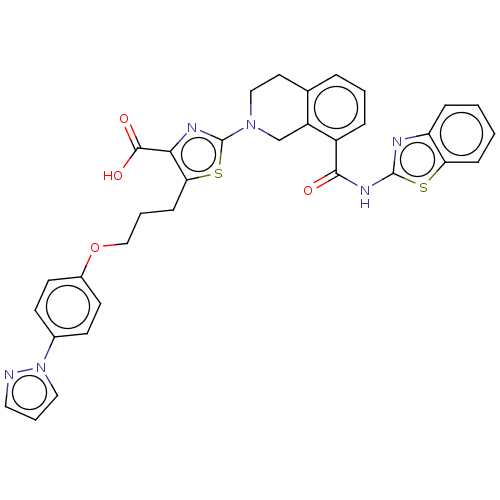
(CHEMBL3342194)Show SMILES OC(=O)c1nc(sc1CCCOc1ccc(cc1)-n1cccn1)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C33H28N6O4S2/c40-30(37-32-35-26-8-1-2-9-27(26)44-32)24-7-3-6-21-15-18-38(20-25(21)24)33-36-29(31(41)42)28(45-33)10-4-19-43-23-13-11-22(12-14-23)39-17-5-16-34-39/h1-3,5-9,11-14,16-17H,4,10,15,18-20H2,(H,41,42)(H,35,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030758
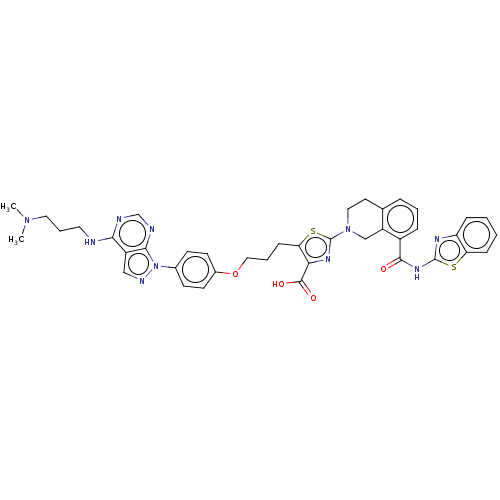
(CHEMBL3342195)Show SMILES CN(C)CCCNc1ncnc2n(ncc12)-c1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C40H40N10O4S2/c1-48(2)19-7-18-41-35-29-22-44-50(36(29)43-24-42-35)26-13-15-27(16-14-26)54-21-6-12-33-34(38(52)53)46-40(56-33)49-20-17-25-8-5-9-28(30(25)23-49)37(51)47-39-45-31-10-3-4-11-32(31)55-39/h3-5,8-11,13-16,22,24H,6-7,12,17-21,23H2,1-2H3,(H,52,53)(H,41,42,43)(H,45,47,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030757
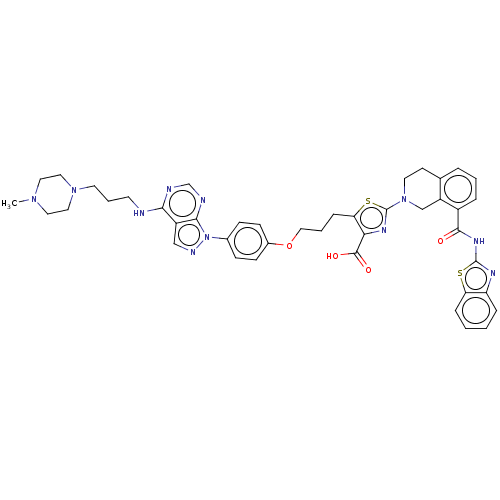
(CHEMBL3342196)Show SMILES CN1CCN(CCCNc2ncnc3n(ncc23)-c2ccc(OCCCc3sc(nc3C(O)=O)N3CCc4cccc(C(=O)Nc5nc6ccccc6s5)c4C3)cc2)CC1 Show InChI InChI=1S/C43H45N11O4S2/c1-51-20-22-52(23-21-51)18-6-17-44-38-32-25-47-54(39(32)46-27-45-38)29-12-14-30(15-13-29)58-24-5-11-36-37(41(56)57)49-43(60-36)53-19-16-28-7-4-8-31(33(28)26-53)40(55)50-42-48-34-9-2-3-10-35(34)59-42/h2-4,7-10,12-15,25,27H,5-6,11,16-24,26H2,1H3,(H,56,57)(H,44,45,46)(H,48,50,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030754
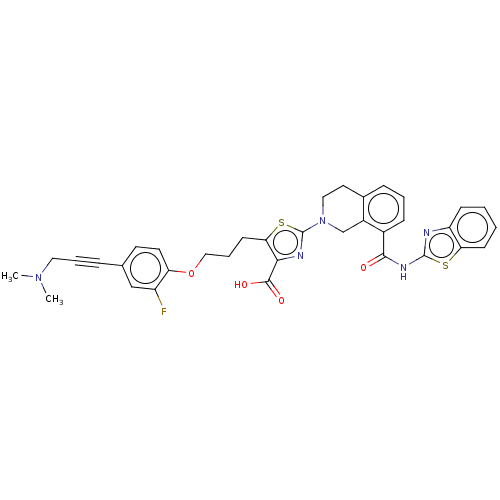
(CHEMBL3342332)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)c(F)c1 Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-8-22-14-15-28(26(36)20-22)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-23-9-5-10-24(25(23)21-41)32(42)39-34-37-27-11-3-4-12-29(27)46-34/h3-5,9-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030752
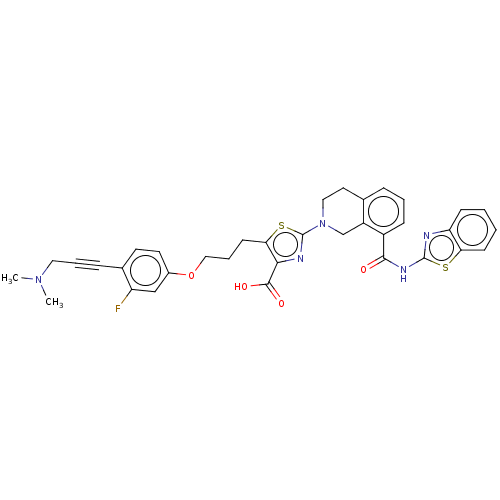
(CHEMBL3342333)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1F Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-9-23-14-15-24(20-27(23)36)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-22-8-5-10-25(26(22)21-41)32(42)39-34-37-28-11-3-4-12-29(28)46-34/h3-5,8,10-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50093275
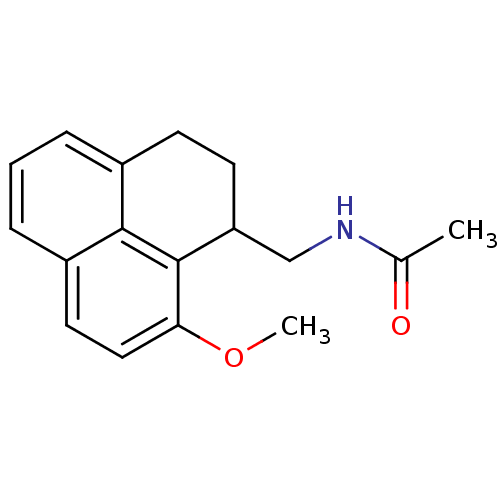
(CHEMBL132802 | N-(9-Methoxy-2,3-dihydro-1H-phenale...)Show InChI InChI=1S/C17H19NO2/c1-11(19)18-10-14-7-6-12-4-3-5-13-8-9-15(20-2)17(14)16(12)13/h3-5,8-9,14H,6-7,10H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Binding affinity for human melatonin receptor type 1A, expressed in HEK-293 cells (2-[125I]-Iodomelatonin is used as radioligand) |
J Med Chem 43: 4051-62 (2000)
BindingDB Entry DOI: 10.7270/Q20Z72JX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50594821
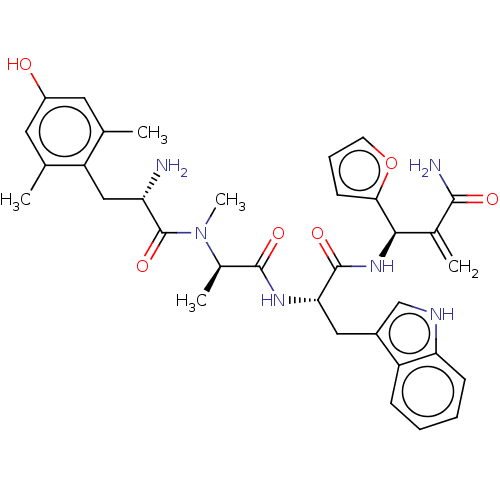
(CHEMBL5175179)Show SMILES C[C@@H](N(C)C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@H](C(=C)C(N)=O)c1ccco1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50093293

(CHEMBL336054 | N-(4,9-Dimethoxy-2,3-dihydro-1H-phe...)Show InChI InChI=1S/C19H23NO3/c1-4-17(21)20-11-13-5-8-14-15(22-2)9-6-12-7-10-16(23-3)19(13)18(12)14/h6-7,9-10,13H,4-5,8,11H2,1-3H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud
Curated by ChEMBL
| Assay Description
Binding affinity for human melatonin receptor type 1A, expressed in HEK-293 cells (2-[125I]-Iodomelatonin is used as radioligand) |
J Med Chem 43: 4051-62 (2000)
BindingDB Entry DOI: 10.7270/Q20Z72JX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data