 Found 194 hits with Last Name = 'pont' and Initial = 'c'
Found 194 hits with Last Name = 'pont' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50062599
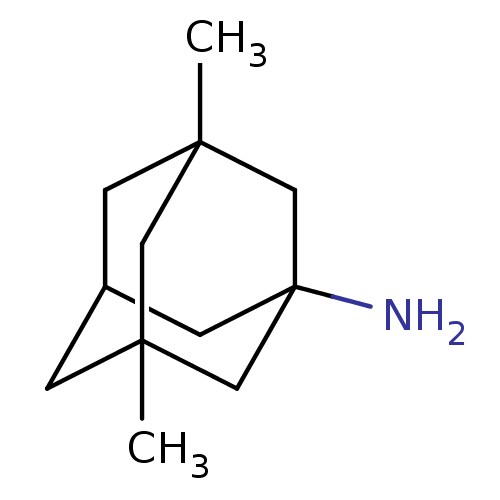
(3,5-Dimethyl-adamantan-1-ylamine | CHEMBL807 | EN3...)Show SMILES CC12CC3CC(C)(C1)CC(N)(C3)C2 |TLB:7:1:4.5.8:11,10:9:4:2.7.1,0:1:4:8.9.11,THB:7:5:11:2.1.12,12:1:4:8.9.11,12:9:4:2.7.1,6:5:11:2.1.12| Show InChI InChI=1S/C12H21N/c1-10-3-9-4-11(2,6-10)8-12(13,5-9)7-10/h9H,3-8,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GluN1/GluN2B receptor (unknown origin) expressed in HEK293 cells by patch-clamp method |
Eur J Med Chem 180: 613-626 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.051
BindingDB Entry DOI: 10.7270/Q2P55RRD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50502562
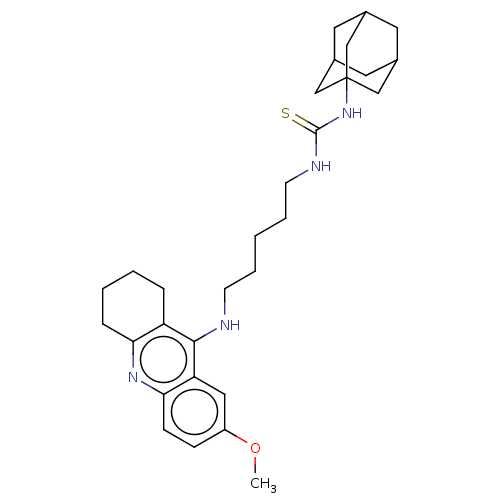
(CHEMBL4469066)Show SMILES COc1ccc2nc3CCCCc3c(NCCCCCNC(=S)NC34CC5CC(CC(C5)C3)C4)c2c1 |TLB:33:24:31:28.27.29,33:28:24.25.32:31,23:24:31:28.27.29,THB:29:28:25:30.32.31,29:30:25:28.33.27| Show InChI InChI=1S/C30H42N4OS/c1-35-23-9-10-27-25(16-23)28(24-7-3-4-8-26(24)33-27)31-11-5-2-6-12-32-29(36)34-30-17-20-13-21(18-30)15-22(14-20)19-30/h9-10,16,20-22H,2-8,11-15,17-19H2,1H3,(H,31,33)(H2,32,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GluN1/GluN2B receptor (unknown origin) expressed in HEK293 cells by patch-clamp method |
Eur J Med Chem 180: 613-626 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.051
BindingDB Entry DOI: 10.7270/Q2P55RRD |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Homo sapiens (Human)) | BDBM50292582
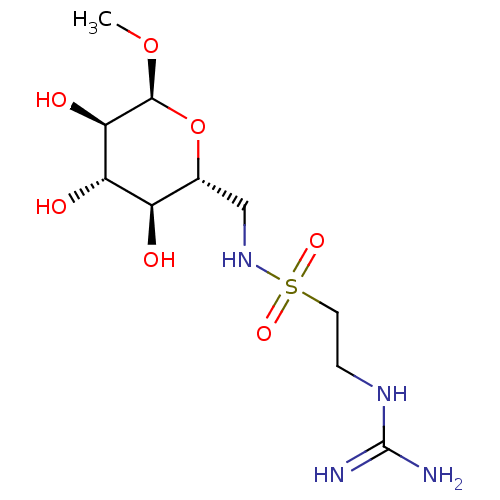
(CHEMBL4162427)Show SMILES CO[C@H]1O[C@H](CNS(=O)(=O)CCNC(N)=N)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H22N4O7S/c1-20-9-8(17)7(16)6(15)5(21-9)4-14-22(18,19)3-2-13-10(11)12/h5-9,14-17H,2-4H2,1H3,(H4,11,12,13)/t5-,6-,7+,8-,9+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Homo sapiens (Human)) | BDBM50292584

(CHEMBL4172694)Show SMILES CO[C@H]1O[C@H](CS(=O)(=O)CCCNC(N)=N)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H23N3O7S/c1-20-10-9(17)8(16)7(15)6(21-10)5-22(18,19)4-2-3-14-11(12)13/h6-10,15-17H,2-5H2,1H3,(H4,12,13,14)/t6-,7-,8+,9-,10+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase
(Plasmodium falciparum (isolate 3D7)) | BDBM50292583

(CHEMBL4164794)Show SMILES CO[C@H]1O[C@H](CNS(=O)(=O)CCN)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C9H20N2O7S/c1-17-9-8(14)7(13)6(12)5(18-9)4-11-19(15,16)3-2-10/h5-9,11-14H,2-4,10H2,1H3/t5-,6-,7+,8-,9+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase
(Plasmodium falciparum (isolate 3D7)) | BDBM50292582

(CHEMBL4162427)Show SMILES CO[C@H]1O[C@H](CNS(=O)(=O)CCNC(N)=N)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H22N4O7S/c1-20-9-8(17)7(16)6(15)5(21-9)4-14-22(18,19)3-2-13-10(11)12/h5-9,14-17H,2-4H2,1H3,(H4,11,12,13)/t5-,6-,7+,8-,9+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase
(Plasmodium falciparum (isolate 3D7)) | BDBM50292585

(CHEMBL4161382)Show SMILES CO[C@H]1O[C@H](CS(=O)(=O)CCCN)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21NO7S/c1-17-10-9(14)8(13)7(12)6(18-10)5-19(15,16)4-2-3-11/h6-10,12-14H,2-5,11H2,1H3/t6-,7-,8+,9-,10+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Homo sapiens (Human)) | BDBM50292585

(CHEMBL4161382)Show SMILES CO[C@H]1O[C@H](CS(=O)(=O)CCCN)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21NO7S/c1-17-10-9(14)8(13)7(12)6(18-10)5-19(15,16)4-2-3-11/h6-10,12-14H,2-5,11H2,1H3/t6-,7-,8+,9-,10+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of VLA-4 expressed in Jurkat cell line, in a cell-based adhesion assay. |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase
(Plasmodium falciparum (isolate 3D7)) | BDBM50292584

(CHEMBL4172694)Show SMILES CO[C@H]1O[C@H](CS(=O)(=O)CCCNC(N)=N)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H23N3O7S/c1-20-10-9(17)8(16)7(15)6(21-10)5-22(18,19)4-2-3-14-11(12)13/h6-10,15-17H,2-5H2,1H3,(H4,12,13,14)/t6-,7-,8+,9-,10+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase
(Plasmodium falciparum (isolate 3D7)) | BDBM50292590

(CHEMBL4162165)Show SMILES CO[C@H]1O[C@H](CSCCNC(N)=N)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21N3O5S/c1-17-9-8(16)7(15)6(14)5(18-9)4-19-3-2-13-10(11)12/h5-9,14-16H,2-4H2,1H3,(H4,11,12,13)/t5-,6-,7+,8-,9+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase
(Plasmodium falciparum (isolate 3D7)) | BDBM50292586
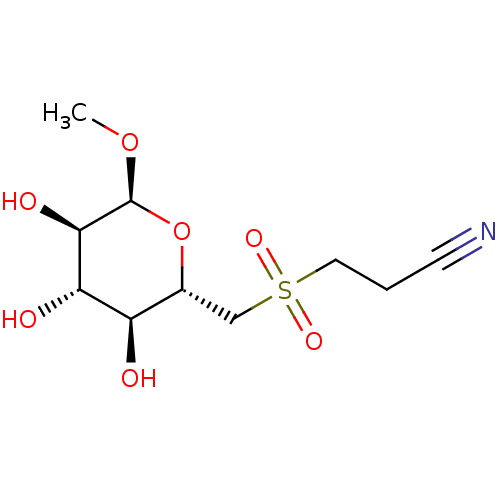
(CHEMBL4169301)Show SMILES CO[C@H]1O[C@H](CS(=O)(=O)CCC#N)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H17NO7S/c1-17-10-9(14)8(13)7(12)6(18-10)5-19(15,16)4-2-3-11/h6-10,12-14H,2,4-5H2,1H3/t6-,7-,8+,9-,10+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase
(Plasmodium falciparum (isolate 3D7)) | BDBM50292580

(CHEMBL4172838)Show SMILES CO[C@H]1O[C@H](CSCCN)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C9H19NO5S/c1-14-9-8(13)7(12)6(11)5(15-9)4-16-3-2-10/h5-9,11-13H,2-4,10H2,1H3/t5-,6-,7+,8-,9+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Homo sapiens (Human)) | BDBM50292587

(CHEMBL4167547)Show SMILES CO[C@H]1O[C@H](CSCCCN)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21NO5S/c1-15-10-9(14)8(13)7(12)6(16-10)5-17-4-2-3-11/h6-10,12-14H,2-5,11H2,1H3/t6-,7-,8+,9-,10+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Homo sapiens (Human)) | BDBM50292590

(CHEMBL4162165)Show SMILES CO[C@H]1O[C@H](CSCCNC(N)=N)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21N3O5S/c1-17-9-8(16)7(15)6(14)5(18-9)4-19-3-2-13-10(11)12/h5-9,14-16H,2-4H2,1H3,(H4,11,12,13)/t5-,6-,7+,8-,9+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Homo sapiens (Human)) | BDBM50292589

(CHEMBL4159624)Show SMILES CO[C@H]1O[C@H](CSCCC#N)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H17NO5S/c1-15-10-9(14)8(13)7(12)6(16-10)5-17-4-2-3-11/h6-10,12-14H,2,4-5H2,1H3/t6-,7-,8+,9-,10+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Homo sapiens (Human)) | BDBM50292586

(CHEMBL4169301)Show SMILES CO[C@H]1O[C@H](CS(=O)(=O)CCC#N)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H17NO7S/c1-17-10-9(14)8(13)7(12)6(18-10)5-19(15,16)4-2-3-11/h6-10,12-14H,2,4-5H2,1H3/t6-,7-,8+,9-,10+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase
(Plasmodium falciparum (isolate 3D7)) | BDBM50292589

(CHEMBL4159624)Show SMILES CO[C@H]1O[C@H](CSCCC#N)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H17NO5S/c1-15-10-9(14)8(13)7(12)6(16-10)5-17-4-2-3-11/h6-10,12-14H,2,4-5H2,1H3/t6-,7-,8+,9-,10+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase
(Plasmodium falciparum (isolate 3D7)) | BDBM50292587

(CHEMBL4167547)Show SMILES CO[C@H]1O[C@H](CSCCCN)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21NO5S/c1-15-10-9(14)8(13)7(12)6(16-10)5-17-4-2-3-11/h6-10,12-14H,2-5,11H2,1H3/t6-,7-,8+,9-,10+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Homo sapiens (Human)) | BDBM50292583

(CHEMBL4164794)Show SMILES CO[C@H]1O[C@H](CNS(=O)(=O)CCN)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C9H20N2O7S/c1-17-9-8(14)7(13)6(12)5(18-9)4-11-19(15,16)3-2-10/h5-9,11-14H,2-4,10H2,1H3/t5-,6-,7+,8-,9+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Homo sapiens (Human)) | BDBM50292580

(CHEMBL4172838)Show SMILES CO[C@H]1O[C@H](CSCCN)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C9H19NO5S/c1-14-9-8(13)7(12)6(11)5(15-9)4-16-3-2-10/h5-9,11-13H,2-4,10H2,1H3/t5-,6-,7+,8-,9+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... |
Eur J Med Chem 146: 108-122 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.044
BindingDB Entry DOI: 10.7270/Q2CN76FJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50502563

(CHEMBL4471659)Show SMILES NC12CC3CC(F)(CC(C1)c1cc(NC(=O)CCCNc4c5CCCCc5nc5cc(Cl)ccc45)ccc31)C2 |TLB:10:8:3.2.4:38,11:10:9:5.7.38,THB:36:37:9:5.7.38,4:3:9:5.7.38,4:5:9:3.2.37.10| Show InChI InChI=1S/C32H36ClFN4O/c33-21-7-9-25-28(12-21)38-27-5-2-1-4-24(27)30(25)36-11-3-6-29(39)37-22-8-10-23-19-14-31(34)15-20(26(23)13-22)17-32(35,16-19)18-31/h7-10,12-13,19-20H,1-6,11,14-18,35H2,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 20 mins followed by subst... |
Eur J Med Chem 180: 613-626 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.051
BindingDB Entry DOI: 10.7270/Q2P55RRD |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50604191
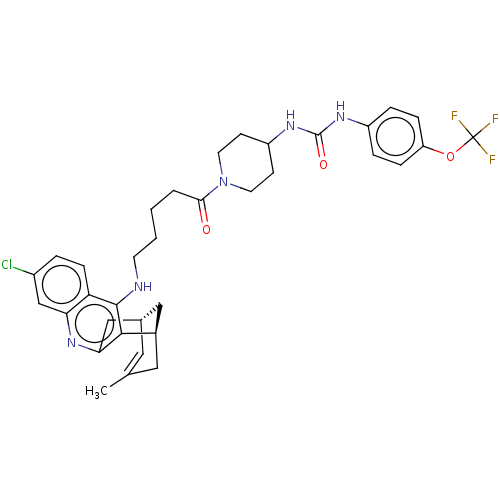
(CHEMBL5207628)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCC(=O)N4CCC(CC4)NC(=O)Nc4ccc(OC(F)(F)F)cc4)c3[C@]([H])(CC(C)=C1)C2 |r,c:50| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50604188
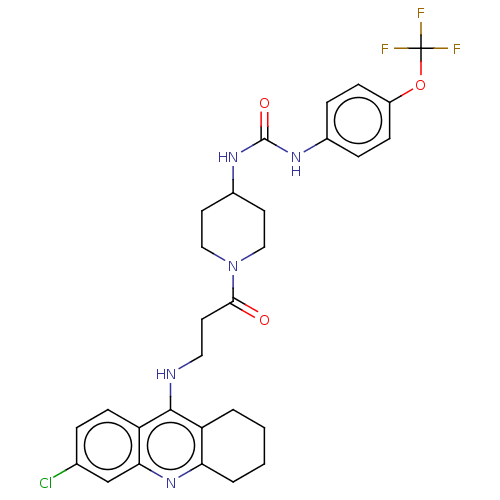
(CHEMBL5204900)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)CCNc2c3CCCCc3nc3cc(Cl)ccc23)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579160
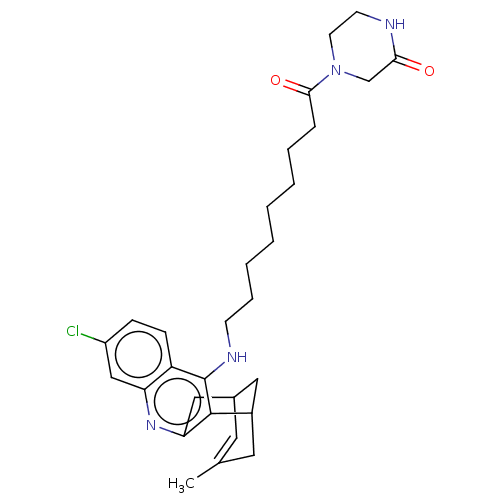
(CHEMBL4854913)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCCC(=O)N1CCNC(=O)C1 |t:1| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113779
BindingDB Entry DOI: 10.7270/Q2TB1BQF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50379273

(CHEMBL1994202 | US9238626, (-)-Huprine Y HCl)Show SMILES CC1=C[C@H]2C[C@@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:1| Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20)/t10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579161

(CHEMBL4848527)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCCC(=O)NCc1cc[nH]c(=O)c1 |t:1| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113779
BindingDB Entry DOI: 10.7270/Q2TB1BQF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50379273

(CHEMBL1994202 | US9238626, (-)-Huprine Y HCl)Show SMILES CC1=C[C@H]2C[C@@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:1| Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20)/t10-,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50604189

(CHEMBL5200047)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)CCCNc2c3CCCCc3nc3cc(Cl)ccc23)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10592

(7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...)Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113779
BindingDB Entry DOI: 10.7270/Q2TB1BQF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579162

(CHEMBL4872514)Show SMILES COc1cc(CNC(=O)CCCCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccn1 |t:36| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113779
BindingDB Entry DOI: 10.7270/Q2TB1BQF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50502565
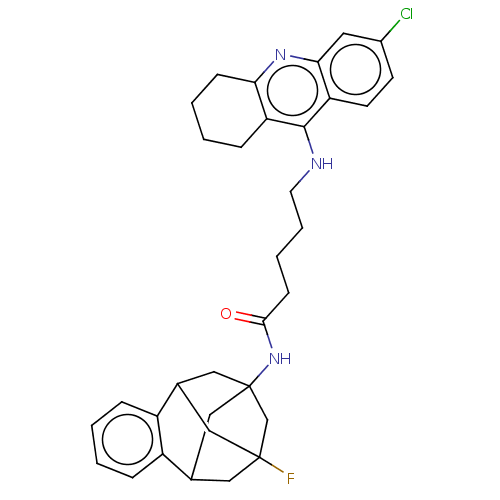
(CHEMBL4462369)Show SMILES FC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)CCCCNc1c2CCCCc2nc2cc(Cl)ccc12 |TLB:14:3:7.6.8:15,13:14:4:1.2.15,THB:8:7:4:1.2.15,8:1:4:7.6.9.14,10:9:4:1.2.15| Show InChI InChI=1S/C33H37ClFN3O/c34-23-12-13-27-29(15-23)37-28-10-4-3-9-26(28)31(27)36-14-6-5-11-30(39)38-33-18-21-16-32(35,20-33)17-22(19-33)25-8-2-1-7-24(21)25/h1-2,7-8,12-13,15,21-22H,3-6,9-11,14,16-20H2,(H,36,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 20 mins followed by subst... |
Eur J Med Chem 180: 613-626 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.051
BindingDB Entry DOI: 10.7270/Q2P55RRD |
More data for this
Ligand-Target Pair | |
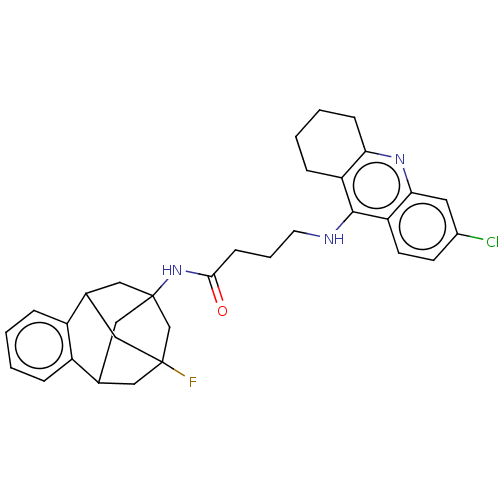
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50502566
(CHEMBL4438626)Show SMILES FC12CC3CC(CC(C1)c1ccccc31)(C2)NC(=O)CCCNc1c2CCCCc2nc2cc(Cl)ccc12 |TLB:14:3:7.6.8:15,13:14:4:1.2.15,THB:8:7:4:1.2.15,8:1:4:7.6.9.14,10:9:4:1.2.15| Show InChI InChI=1S/C32H35ClFN3O/c33-22-11-12-26-28(14-22)36-27-9-4-3-8-25(27)30(26)35-13-5-10-29(38)37-32-17-20-15-31(34,19-32)16-21(18-32)24-7-2-1-6-23(20)24/h1-2,6-7,11-12,14,20-21H,3-5,8-10,13,15-19H2,(H,35,36)(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 20 mins followed by subst... |
Eur J Med Chem 180: 613-626 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.051
BindingDB Entry DOI: 10.7270/Q2P55RRD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50502564

(CHEMBL4558495)Show SMILES FC12CC3CC(CC(C1)c1ccccc31)(C2)NCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 |TLB:14:3:7.6.8:15,13:14:4:1.2.15,THB:8:7:4:1.2.15,8:1:4:7.6.9.14,10:9:4:1.2.15| Show InChI InChI=1S/C33H39ClFN3/c34-24-12-13-28-30(16-24)38-29-11-5-4-10-27(29)31(28)36-14-6-1-7-15-37-33-19-22-17-32(35,21-33)18-23(20-33)26-9-3-2-8-25(22)26/h2-3,8-9,12-13,16,22-23,37H,1,4-7,10-11,14-15,17-21H2,(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 20 mins followed by subst... |
Eur J Med Chem 180: 613-626 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.051
BindingDB Entry DOI: 10.7270/Q2P55RRD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579158

(CHEMBL4866930)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCCCNC(=O)c1ccc[nH]c1=O |t:1| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113779
BindingDB Entry DOI: 10.7270/Q2TB1BQF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50604191

(CHEMBL5207628)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCC(=O)N4CCC(CC4)NC(=O)Nc4ccc(OC(F)(F)F)cc4)c3[C@]([H])(CC(C)=C1)C2 |r,c:50| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
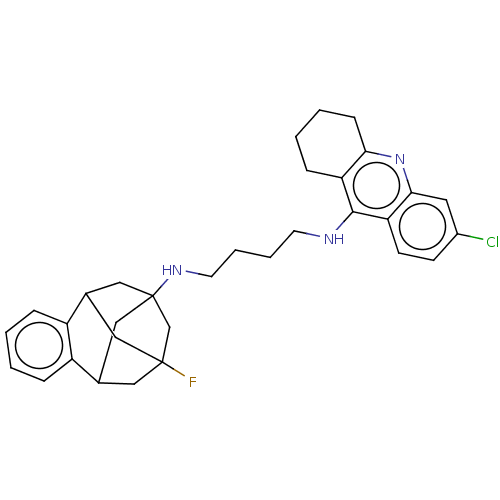
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50502567
(CHEMBL4469343)Show SMILES FC12CC3CC(CC(C1)c1ccccc31)(C2)NCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 |TLB:14:3:7.6.8:15,13:14:4:1.2.15,THB:8:7:4:1.2.15,8:1:4:7.6.9.14,10:9:4:1.2.15| Show InChI InChI=1S/C32H37ClFN3/c33-23-11-12-27-29(15-23)37-28-10-4-3-9-26(28)30(27)35-13-5-6-14-36-32-18-21-16-31(34,20-32)17-22(19-32)25-8-2-1-7-24(21)25/h1-2,7-8,11-12,15,21-22,36H,3-6,9-10,13-14,16-20H2,(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 20 mins followed by subst... |
Eur J Med Chem 180: 613-626 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.051
BindingDB Entry DOI: 10.7270/Q2P55RRD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579159
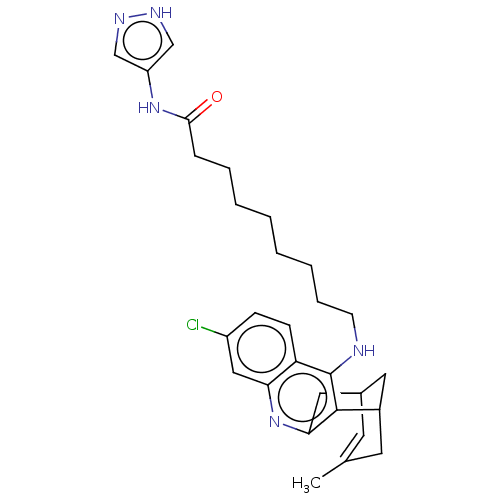
(CHEMBL4863615)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCCC(=O)Nc1cn[nH]c1 |t:1| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113779
BindingDB Entry DOI: 10.7270/Q2TB1BQF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579156
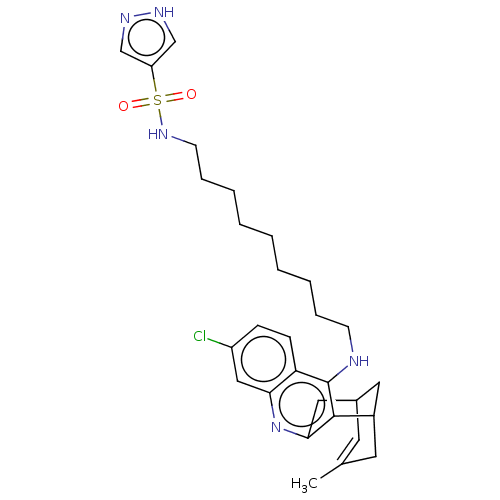
(CHEMBL4862716)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCCCNS(=O)(=O)c1cn[nH]c1 |t:1| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113779
BindingDB Entry DOI: 10.7270/Q2TB1BQF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50502568
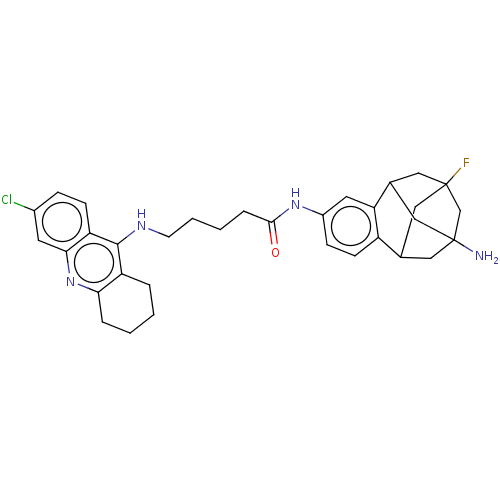
(CHEMBL4591167)Show SMILES NC12CC3CC(F)(CC(C1)c1cc(NC(=O)CCCCNc4c5CCCCc5nc5cc(Cl)ccc45)ccc31)C2 |TLB:10:8:3.2.4:39,11:10:9:5.7.39,THB:37:38:9:5.7.39,4:3:9:5.7.39,4:5:9:3.2.38.10| Show InChI InChI=1S/C33H38ClFN4O/c34-22-8-10-26-29(13-22)39-28-6-2-1-5-25(28)31(26)37-12-4-3-7-30(40)38-23-9-11-24-20-15-32(35)16-21(27(24)14-23)18-33(36,17-20)19-32/h8-11,13-14,20-21H,1-7,12,15-19,36H2,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 20 mins followed by subst... |
Eur J Med Chem 180: 613-626 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.051
BindingDB Entry DOI: 10.7270/Q2P55RRD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50604191

(CHEMBL5207628)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCC(=O)N4CCC(CC4)NC(=O)Nc4ccc(OC(F)(F)F)cc4)c3[C@]([H])(CC(C)=C1)C2 |r,c:50| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50579157

(CHEMBL4859103)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCCCNC(=O)c1cc(O)cc(O)c1 |t:1| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113779
BindingDB Entry DOI: 10.7270/Q2TB1BQF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50604189

(CHEMBL5200047)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)CCCNc2c3CCCCc3nc3cc(Cl)ccc23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM25744
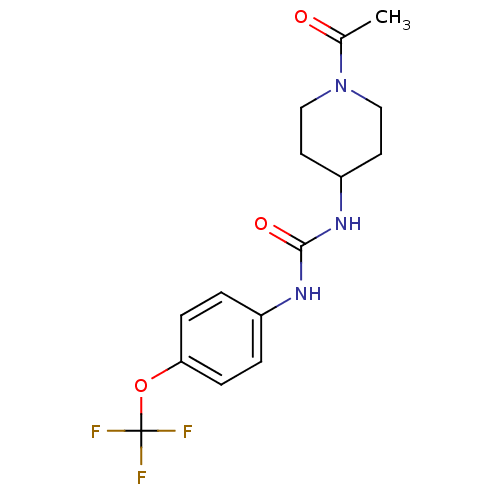
(3-(1-acetylpiperidin-4-yl)-1-[4-(trifluoromethoxy)...)Show InChI InChI=1S/C15H18F3N3O3/c1-10(22)21-8-6-12(7-9-21)20-14(23)19-11-2-4-13(5-3-11)24-15(16,17)18/h2-5,12H,6-9H2,1H3,(H2,19,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13599
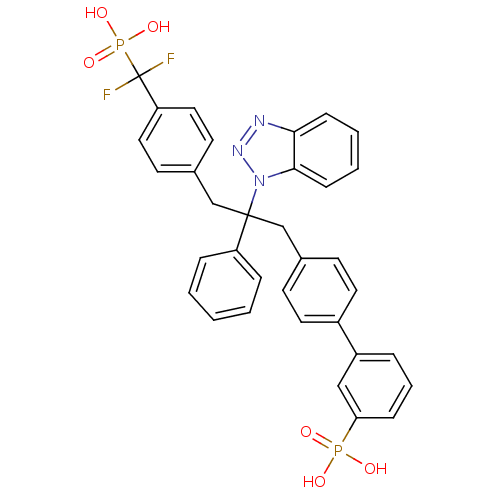
(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13599

(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.3 | 22 |
Merck Research Laboratories
| Assay Description
Hydrolysis of substrate FDP was monitored continuously on a Cytofluor microplate reader with excitation and emission wavelengths set at 440 and 515 ... |
Biochemistry 42: 11451-9 (2003)
Article DOI: 10.1021/bi035098j
BindingDB Entry DOI: 10.7270/Q2HX19XS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50604192
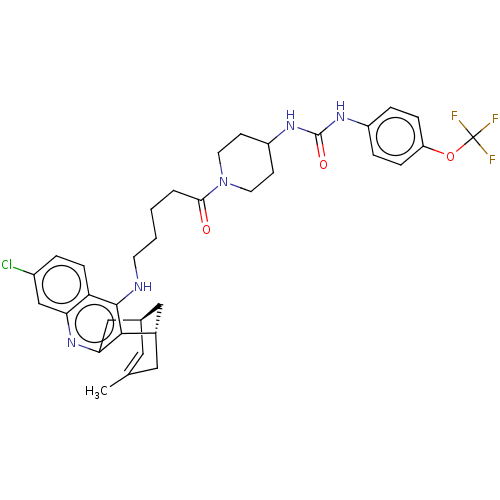
(CHEMBL5188116)Show SMILES [H][C@@]12Cc3nc4cc(Cl)ccc4c(NCCCCC(=O)N4CCC(CC4)NC(=O)Nc4ccc(OC(F)(F)F)cc4)c3[C@@]([H])(CC(C)=C1)C2 |r,c:50| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
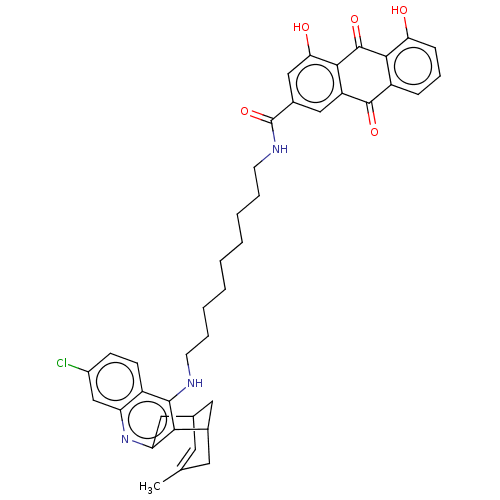
(Homo sapiens (Human)) | BDBM202363
(US9238626, (+/-)-(Ib) HCl)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCCCNC(=O)c1cc(O)c2C(=O)c3c(O)cccc3C(=O)c2c1 |t:1| Show InChI InChI=1S/C41H42ClN3O5/c1-23-16-24-18-25(17-23)35-32(19-24)45-31-22-27(42)12-13-28(31)38(35)43-14-7-5-3-2-4-6-8-15-44-41(50)26-20-30-37(34(47)21-26)40(49)36-29(39(30)48)10-9-11-33(36)46/h9-13,16,20-22,24-25,46-47H,2-8,14-15,17-19H2,1H3,(H,43,45)(H,44,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as a substrate preincubated for 20 mins followed by substrate addition by spectro... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113779
BindingDB Entry DOI: 10.7270/Q2TB1BQF |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25744

(3-(1-acetylpiperidin-4-yl)-1-[4-(trifluoromethoxy)...)Show InChI InChI=1S/C15H18F3N3O3/c1-10(22)21-8-6-12(7-9-21)20-14(23)19-11-2-4-13(5-3-11)24-15(16,17)18/h2-5,12H,6-9H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50604189

(CHEMBL5200047)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)CCCNc2c3CCCCc3nc3cc(Cl)ccc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50604190

(CHEMBL5178885)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)CCCCNc2c3CCCCc3nc3cc(Cl)ccc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data