

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM25744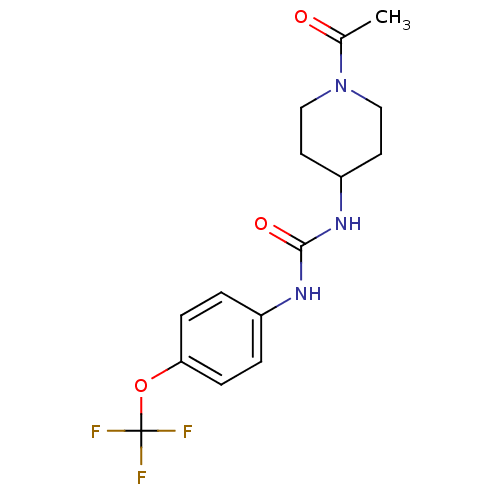 (3-(1-acetylpiperidin-4-yl)-1-[4-(trifluoromethoxy)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02150 BindingDB Entry DOI: 10.7270/Q2F76HMM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM25744 (3-(1-acetylpiperidin-4-yl)-1-[4-(trifluoromethoxy)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02150 BindingDB Entry DOI: 10.7270/Q2F76HMM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM25744 (3-(1-acetylpiperidin-4-yl)-1-[4-(trifluoromethoxy)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase | Bioorg Med Chem Lett 21: 983-8 (2011) Article DOI: 10.1016/j.bmcl.2010.12.042 BindingDB Entry DOI: 10.7270/Q20865KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM25744 (3-(1-acetylpiperidin-4-yl)-1-[4-(trifluoromethoxy)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET | Bioorg Med Chem Lett 21: 983-8 (2011) Article DOI: 10.1016/j.bmcl.2010.12.042 BindingDB Entry DOI: 10.7270/Q20865KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM25744 (3-(1-acetylpiperidin-4-yl)-1-[4-(trifluoromethoxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of rat soluble epoxide hydrolase using [3H]-t-DPPO as a substrate by radiometric assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Sus scrofa (Pig)) | BDBM25744 (3-(1-acetylpiperidin-4-yl)-1-[4-(trifluoromethoxy)...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
THE REGENTS OF THE UNIVERSITY OF CALIFORNIA US Patent | Assay Description The enzyme also can be detected based on the binding of specific ligands to the catalytic site which either immobilize the enzyme or label it with a ... | US Patent US10383835 (2019) BindingDB Entry DOI: 10.7270/Q2XD141M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||