

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T... | Eur J Med Chem 131: 107-125 (2017) Article DOI: 10.1016/j.ejmech.2017.03.001 BindingDB Entry DOI: 10.7270/Q21V5HCK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50245603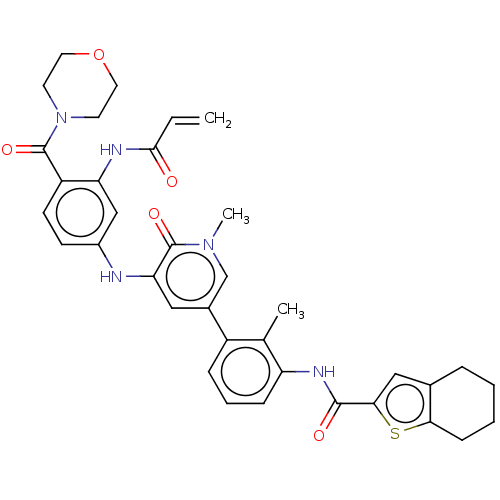 (CHEMBL4096207) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T... | Eur J Med Chem 131: 107-125 (2017) Article DOI: 10.1016/j.ejmech.2017.03.001 BindingDB Entry DOI: 10.7270/Q21V5HCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50245586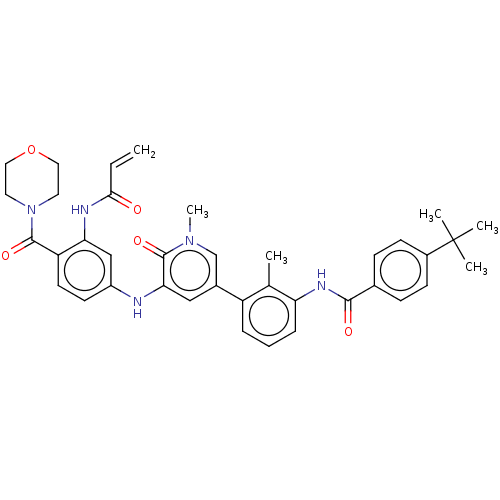 (CHEMBL4077123) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T... | Eur J Med Chem 131: 107-125 (2017) Article DOI: 10.1016/j.ejmech.2017.03.001 BindingDB Entry DOI: 10.7270/Q21V5HCK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50245587 (CHEMBL4077064 | US10000483, Compound II-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T... | Eur J Med Chem 131: 107-125 (2017) Article DOI: 10.1016/j.ejmech.2017.03.001 BindingDB Entry DOI: 10.7270/Q21V5HCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50161162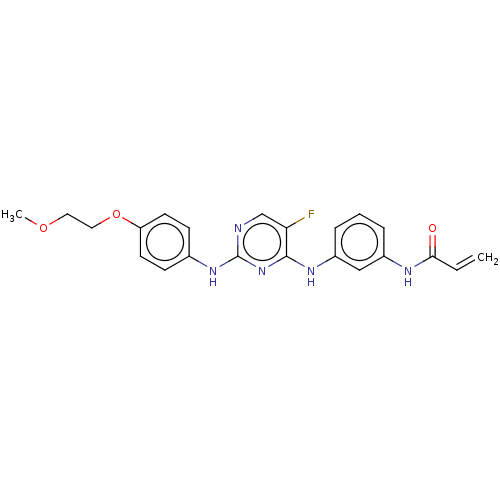 (AVL-292 | CC-292 | Spebrutinib | US10596172, Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T... | Eur J Med Chem 131: 107-125 (2017) Article DOI: 10.1016/j.ejmech.2017.03.001 BindingDB Entry DOI: 10.7270/Q21V5HCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50020471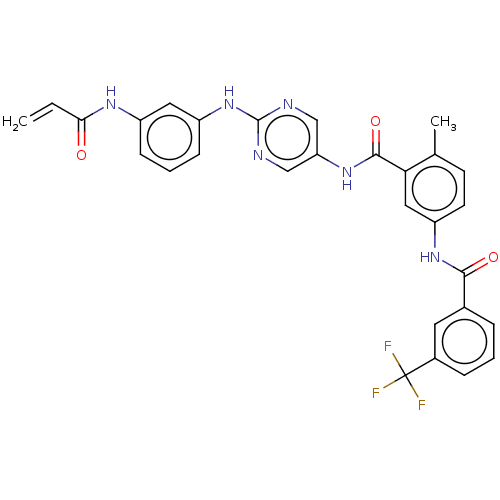 (CHEMBL3290142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T... | Eur J Med Chem 131: 107-125 (2017) Article DOI: 10.1016/j.ejmech.2017.03.001 BindingDB Entry DOI: 10.7270/Q21V5HCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50245588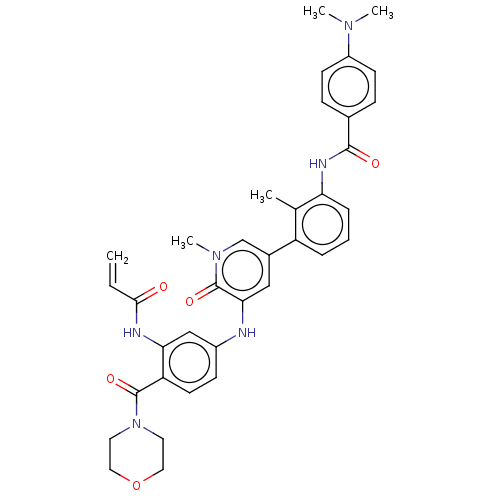 (CHEMBL4085868) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T... | Eur J Med Chem 131: 107-125 (2017) Article DOI: 10.1016/j.ejmech.2017.03.001 BindingDB Entry DOI: 10.7270/Q21V5HCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50403056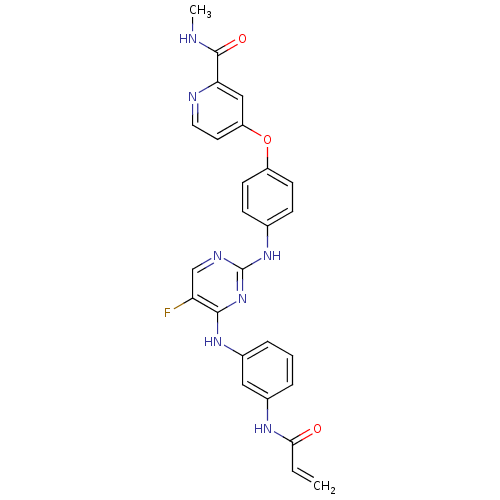 (CHEMBL2216827 | US10596172, Compound I-342 | US108...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology of China Curated by ChEMBL | Assay Description Reversible inhibition of full length recombinant human N-terminal His tagged BKT expressed in baculovirus infected Sf9 insect cells using poly (Glu,T... | Eur J Med Chem 131: 107-125 (2017) Article DOI: 10.1016/j.ejmech.2017.03.001 BindingDB Entry DOI: 10.7270/Q21V5HCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50397078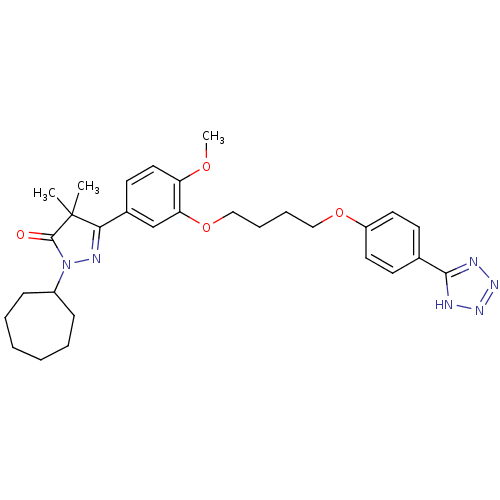 (CHEMBL2171452) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of human DNMT3A using AdoMet as substrate | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127908 BindingDB Entry DOI: 10.7270/Q27H1PCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50397078 (CHEMBL2171452) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human DNMT3A catalytic domain assessed as inhibition of enzyme-mediated DNA methylation using 3H-AdoMet as substrate incubated fo... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127908 BindingDB Entry DOI: 10.7270/Q27H1PCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50427452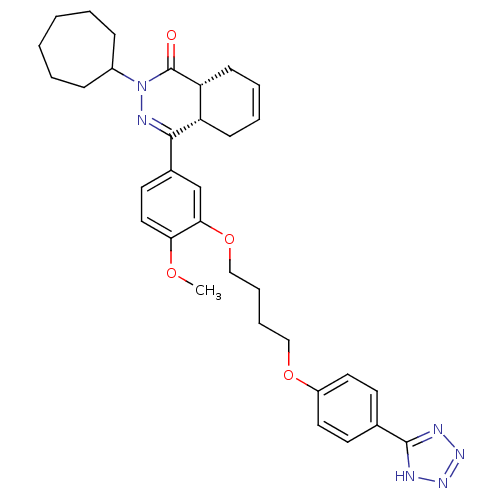 (CHEMBL2326941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 9.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive inhibition of human DNMT3A using AdoMet as substrate | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127908 BindingDB Entry DOI: 10.7270/Q27H1PCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50427452 (CHEMBL2326941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive inhibition of human DNMT3A using poly dl-dC as substrate | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127908 BindingDB Entry DOI: 10.7270/Q27H1PCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50397078 (CHEMBL2171452) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of human DNMT3A using poly dl-dC as substrate | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127908 BindingDB Entry DOI: 10.7270/Q27H1PCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50427452 (CHEMBL2326941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human DNMT3A catalytic domain assessed as inhibition of enzyme-mediated DNA methylation using 3H-AdoMet as substrate incubated fo... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127908 BindingDB Entry DOI: 10.7270/Q27H1PCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50427452 (CHEMBL2326941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human DNMT3A catalytic domain assessed as inhibition of enzyme-mediated DNA methylation using poly dl-dC as substrate incubated f... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127908 BindingDB Entry DOI: 10.7270/Q27H1PCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 3A (Homo sapiens (Human)) | BDBM50397078 (CHEMBL2171452) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human DNMT3A catalytic domain assessed as inhibition of enzyme-mediated DNA methylation using poly dl-dC as substrate incubated f... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127908 BindingDB Entry DOI: 10.7270/Q27H1PCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512366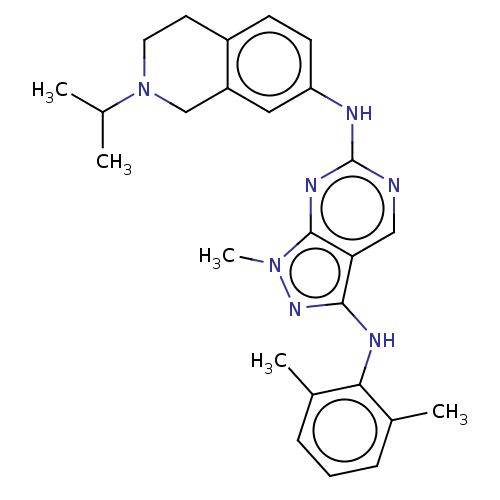 (US11084824, Example 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243386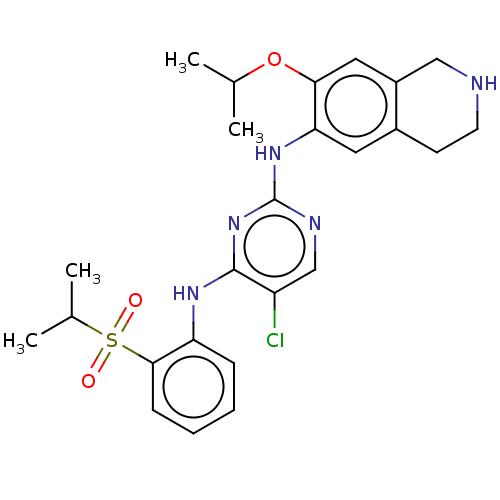 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(7-is...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243402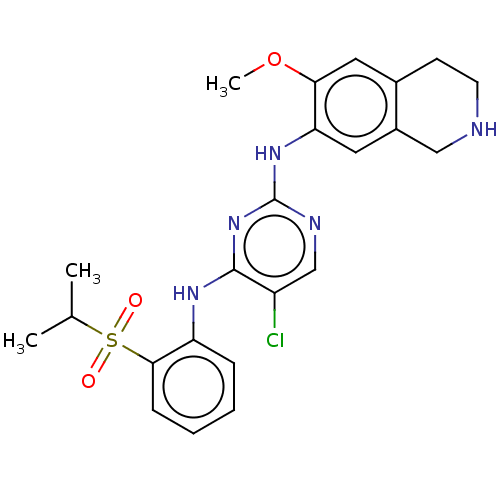 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(6-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of crizotinib-resistant ALK G1269A mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc... | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243393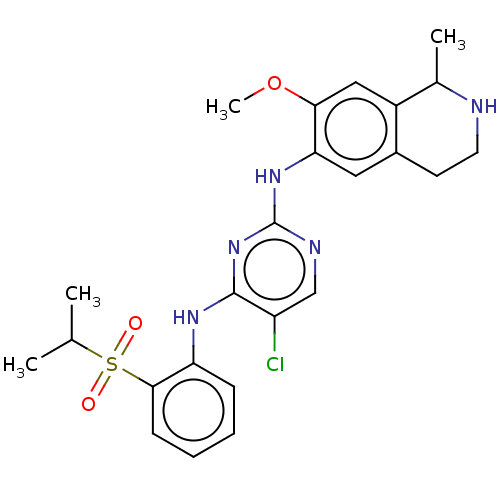 (5-chloro-N4-2-(isopropylsulfonyl)phenyl)-N2-(7-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM243393 (5-chloro-N4-2-(isopropylsulfonyl)phenyl)-N2-(7-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512370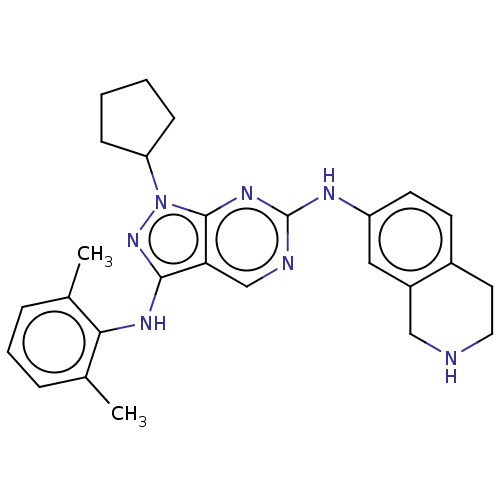 (US11084824, Example 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512369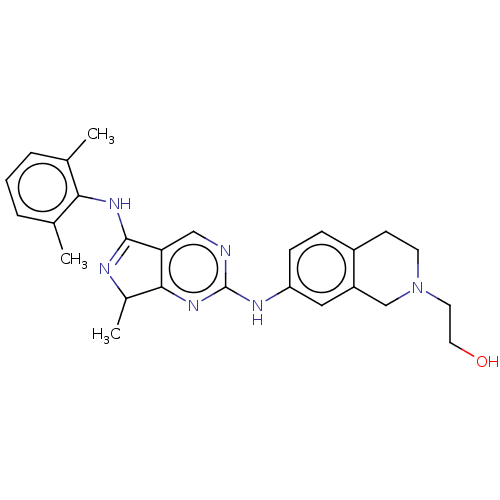 (US11084824, Example 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512364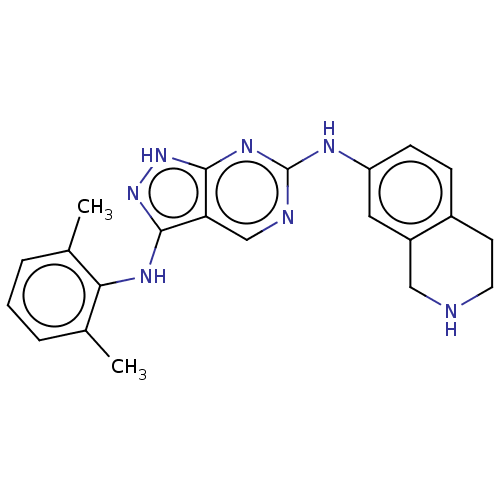 (Preparation of N3-(2,6-dimethylphenyl)-N6-(1,2,3,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512368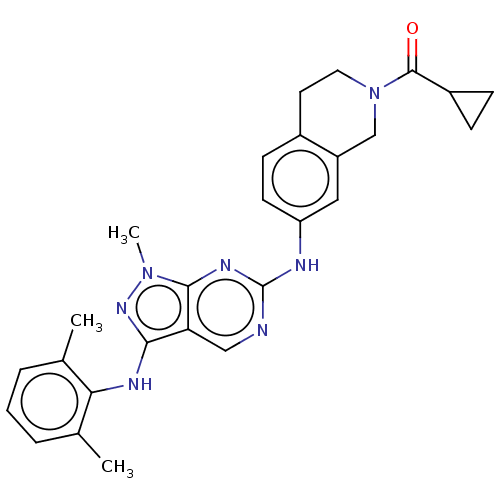 (US11084824, Example 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243395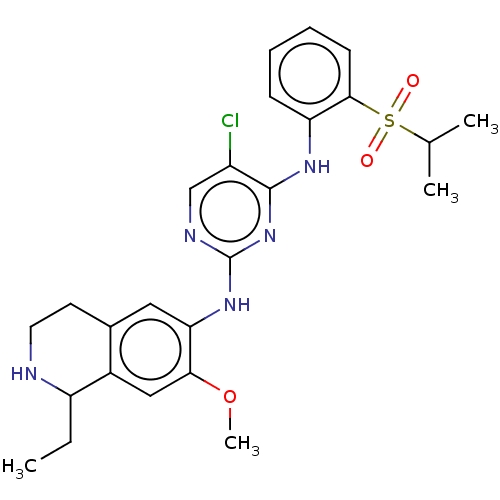 (5-chloro-N4-2-(isopropylsulfonyl)phenyl)-N2-(7-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50240271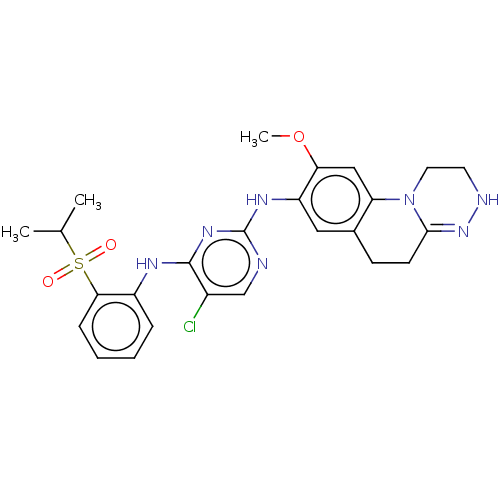 (CHEMBL4101954) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science& Technology Curated by ChEMBL | Assay Description In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes. | Bioorg Med Chem Lett 27: 2185-2191 (2017) Article DOI: 10.1016/j.bmcl.2017.03.073 BindingDB Entry DOI: 10.7270/Q2DR2XNC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50116683 (CHEMBL3608526 | US10053458, 49) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512367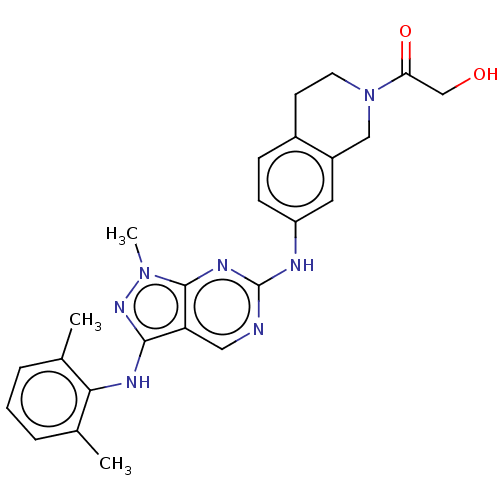 (US11084824, Example 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM291934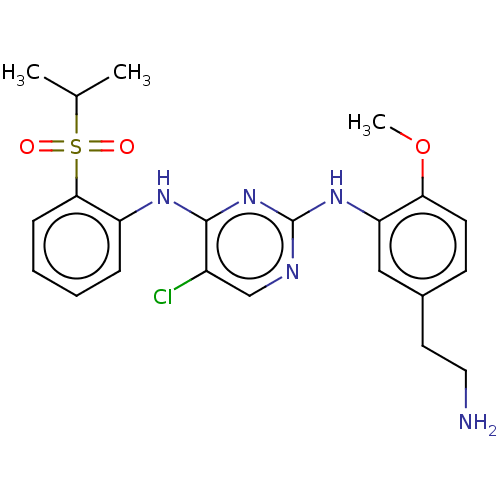 (US10100019, Example 4) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | 25 |
Korea Research Institute of Chemical Technology US Patent | Assay Description The following experiment was performed in order to measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of ... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243402 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(6-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of crizotinib-resistant ALK C1156Y mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc... | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243402 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(6-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of crizotinib-resistant ALK F1174L mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc... | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512351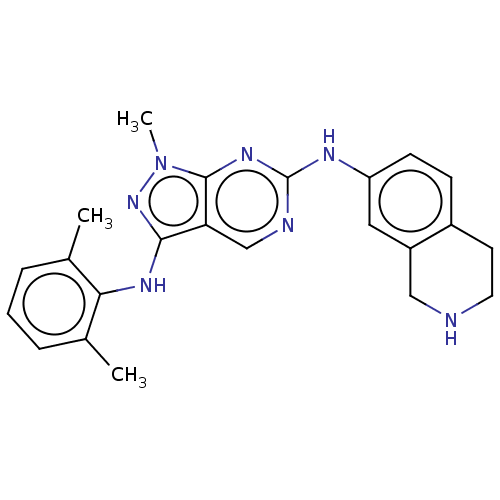 (US11084824, Example 2 | US11084824, Example 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512363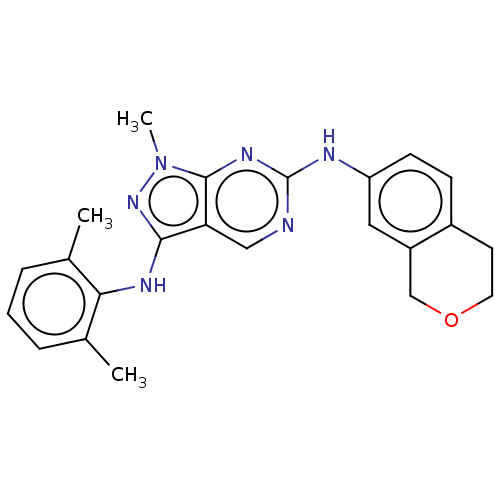 (US11084824, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50157303 (CHEMBL3785774) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of wild type ALK (unknown origin) after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1720-5 (2016) Article DOI: 10.1016/j.bmcl.2016.02.052 BindingDB Entry DOI: 10.7270/Q2TF007P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50116690 (CHEMBL3608528 | US10053458, 67) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512388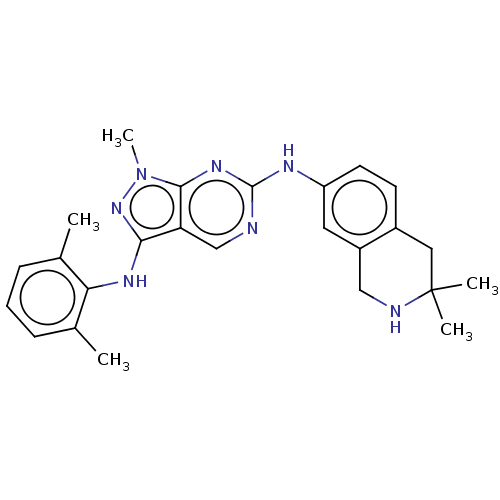 (US11084824, Example 53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.523 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243386 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(7-is...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ALK (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescence assay | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50116696 (CHEMBL3608534 | US10053458, 52) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50582238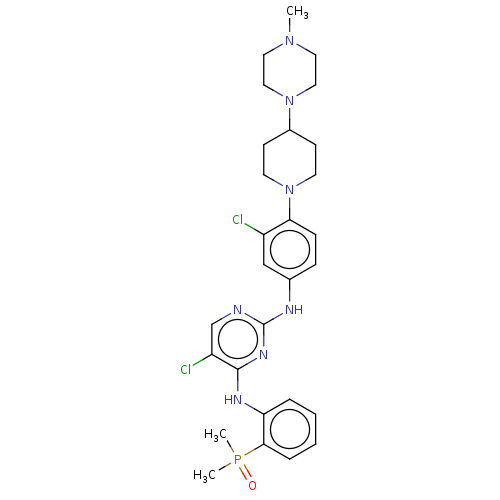 (CHEMBL5076986) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EGFR L858R/T790M/C797S triple mutant (unknown origin) measured by ELISA | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00555 BindingDB Entry DOI: 10.7270/Q2CC14KG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512350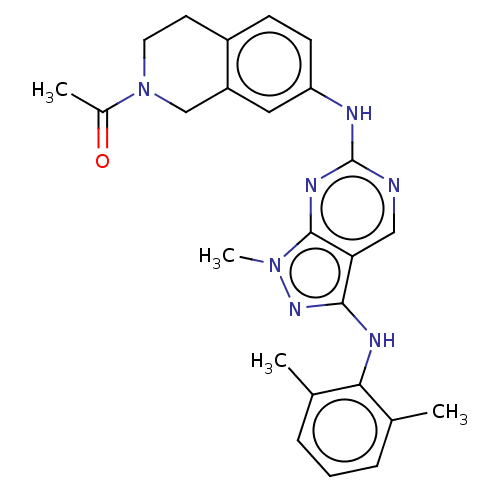 (US11084824, Example 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512351 (US11084824, Example 2 | US11084824, Example 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50116699 (CHEMBL3608642 | US10053458, 53) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512360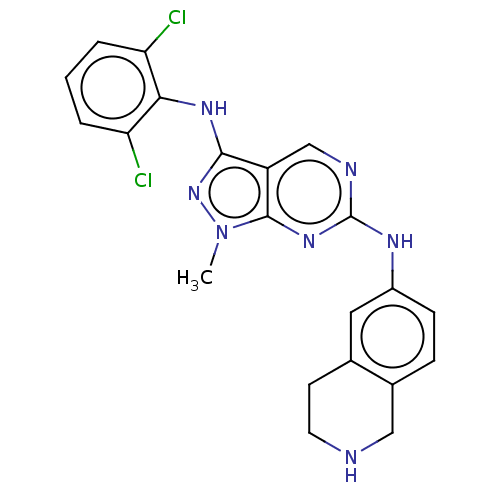 (Preparation of N3-(2,6-dichlorophenyl)-1-methyl-N6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512377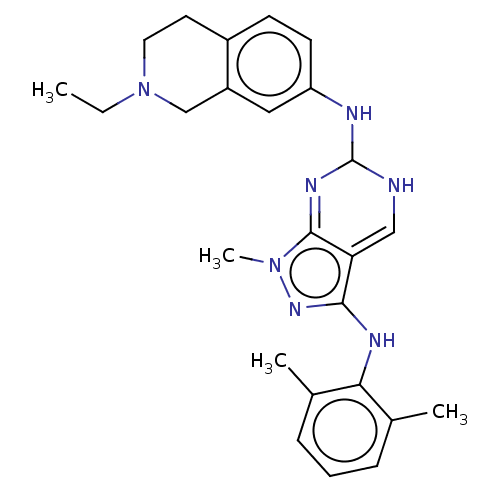 (US11084824, Example 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50157313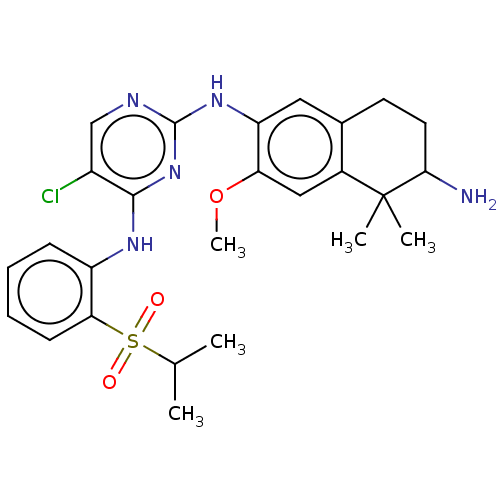 (CHEMBL3786892) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungbuk National University Curated by ChEMBL | Assay Description Inhibition of wild type ALK (unknown origin) after 30 mins by HTRF assay | Bioorg Med Chem Lett 26: 1720-5 (2016) Article DOI: 10.1016/j.bmcl.2016.02.052 BindingDB Entry DOI: 10.7270/Q2TF007P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50582234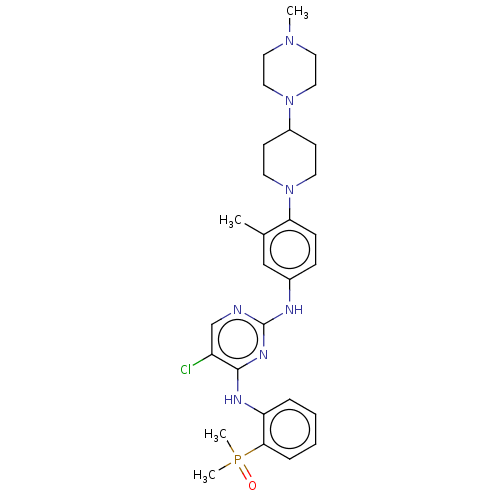 (CHEMBL5084760) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EGFR L858R/T790M/C797S triple mutant (unknown origin) measured by ELISA | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00555 BindingDB Entry DOI: 10.7270/Q2CC14KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50582243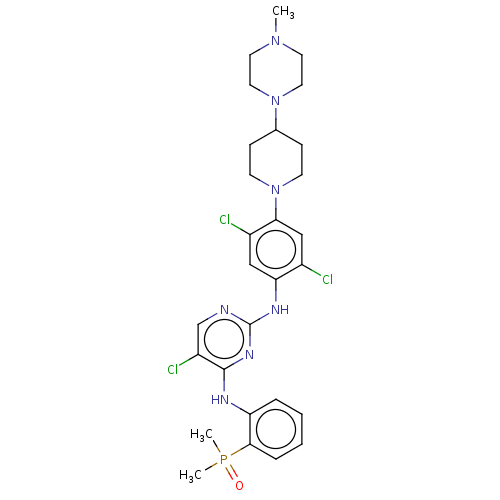 (CHEMBL5081174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EGFR L858R/T790M/C797S triple mutant (unknown origin) measured by ELISA | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00555 BindingDB Entry DOI: 10.7270/Q2CC14KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM50116696 (CHEMBL3608534 | US10053458, 52) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512392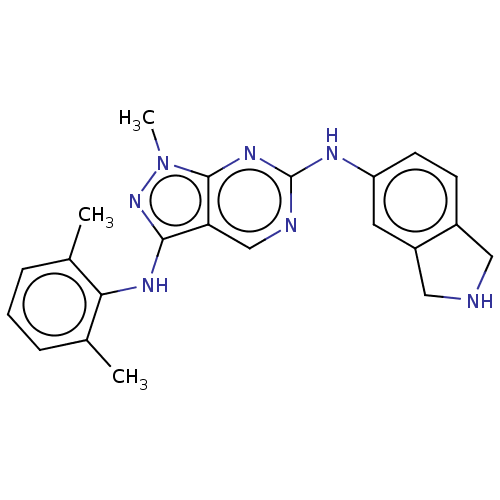 (Preparation of N3-(2,6-dimethylphenyl)-N6-(isoindo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.745 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1210 total ) | Next | Last >> |