 Found 60 hits with Last Name = 'berkman' and Initial = 'ce'
Found 60 hits with Last Name = 'berkman' and Initial = 'ce' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gamma-glutamyl hydrolase
(Homo sapiens (Human)) | BDBM50070541

((2S)-2-{[hydroxy(methyl)phosphoryl]amino}pentanedi...)Show InChI InChI=1S/C6H12NO6P/c1-14(12,13)7-4(6(10)11)2-3-5(8)9/h4H,2-3H2,1H3,(H,8,9)(H,10,11)(H2,7,12,13)/t4-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 9.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory potency against gamma-Glutamyl Hydrolase. |
Bioorg Med Chem Lett 8: 1521-4 (1999)
BindingDB Entry DOI: 10.7270/Q2N015PX |
More data for this
Ligand-Target Pair | |
Gamma-glutamyl hydrolase
(Homo sapiens (Human)) | BDBM50070540

((2S)-2-{[hydroxy(phenyl)phosphoryl]amino}pentanedi...)Show InChI InChI=1S/C11H14NO6P/c13-10(14)7-6-9(11(15)16)12-19(17,18)8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,13,14)(H,15,16)(H2,12,17,18)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory potency against gamma-Glutamyl Hydrolase. |
Bioorg Med Chem Lett 8: 1521-4 (1999)
BindingDB Entry DOI: 10.7270/Q2N015PX |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50358646
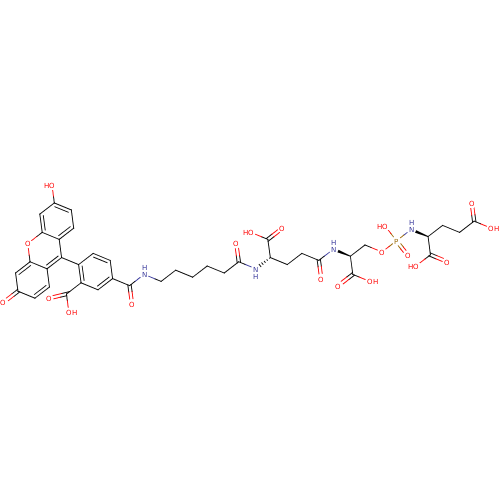
(CHEMBL1921899)Show SMILES OC(=O)CC[C@H](NP(O)(=O)OC[C@H](NC(=O)CC[C@H](NC(=O)CCCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(O)cc2oc2cc(=O)ccc12)C(O)=O)C(O)=O)C(O)=O |r,wD:18.18,12.12,5.5,(26.08,-15.33,;26.07,-13.79,;27.4,-13.02,;24.72,-13.04,;23.39,-13.81,;22.06,-13.06,;20.73,-13.83,;19.39,-13.07,;19.38,-14.61,;18.06,-13.85,;19.38,-11.53,;18.04,-10.77,;18.03,-9.23,;16.69,-8.47,;15.36,-9.24,;15.38,-10.78,;14.02,-8.49,;12.7,-9.26,;11.36,-8.51,;10.03,-9.28,;8.69,-8.52,;8.68,-6.98,;7.36,-9.3,;6.02,-8.54,;4.69,-9.32,;3.36,-8.56,;2.03,-9.34,;.68,-8.58,;-.64,-9.35,;-1.98,-8.6,;-.63,-10.89,;-1.96,-11.68,;-1.95,-13.22,;-.62,-13.97,;.71,-13.2,;.71,-11.66,;2.04,-12.42,;3.39,-13.18,;2.04,-10.88,;-.61,-15.51,;-1.93,-16.3,;-3.27,-15.53,;-4.6,-16.3,;-4.6,-17.85,;-5.93,-18.61,;-3.27,-18.62,;-1.92,-17.84,;-.59,-18.61,;.74,-17.83,;2.07,-18.58,;3.4,-17.81,;4.74,-18.57,;3.39,-16.27,;2.05,-15.51,;.74,-16.28,;11.34,-6.96,;12.68,-6.18,;10.01,-6.2,;19.36,-8.45,;20.7,-9.21,;19.35,-6.91,;22.05,-11.52,;23.38,-10.73,;20.7,-10.75,)| Show InChI InChI=1S/C40H43N4O19P/c45-21-6-9-24-30(17-21)63-31-18-22(46)7-10-25(31)35(24)23-8-5-20(16-26(23)37(52)53)36(51)41-15-3-1-2-4-32(47)42-27(38(54)55)11-13-33(48)43-29(40(58)59)19-62-64(60,61)44-28(39(56)57)12-14-34(49)50/h5-10,16-18,27-29,45H,1-4,11-15,19H2,(H,41,51)(H,42,47)(H,43,48)(H,49,50)(H,52,53)(H,54,55)(H,56,57)(H,58,59)(H2,44,60,61)/t27-,28-,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA using N-[4-(phenylazo)-benzoyl]-glutamyl-gamma-glutamic acid as substrate after 15 mins by HPLC analysis |
Bioorg Med Chem Lett 21: 7013-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.115
BindingDB Entry DOI: 10.7270/Q2PG1S5S |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50358645
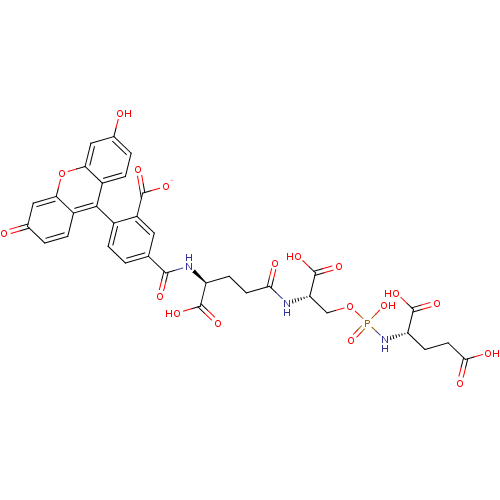
(CHEMBL1921898)Show SMILES OC(=O)CC[C@H](NP(O)(=O)OC[C@H](NC(=O)CC[C@H](NC(=O)c1ccc(c(c1)C([O-])=O)-c1c2ccc(O)cc2oc2cc(=O)ccc12)C(O)=O)C(O)=O)C(O)=O |r,wU:18.18,wD:12.12,5.5,(11.85,-15.93,;11.85,-14.39,;13.19,-13.62,;10.52,-13.62,;9.19,-14.39,;7.85,-13.62,;6.52,-14.39,;5.19,-13.63,;5.17,-15.16,;3.86,-14.4,;5.19,-12.09,;3.86,-11.32,;3.86,-9.78,;2.52,-9.01,;1.19,-9.78,;1.19,-11.32,;-.14,-9.02,;-1.48,-9.79,;-2.81,-9.02,;-2.81,-7.48,;-4.14,-6.71,;-5.47,-7.48,;-4.14,-5.17,;-5.48,-4.4,;-5.48,-2.87,;-4.14,-2.09,;-2.82,-2.86,;-2.81,-4.39,;-1.48,-2.09,;-.15,-2.85,;-2.58,-.99,;-4.14,-.56,;-5.48,.21,;-6.81,-.55,;-8.14,.22,;-8.14,1.76,;-9.47,2.53,;-6.81,2.53,;-5.48,1.77,;-4.14,2.55,;-2.79,1.77,;-1.46,2.54,;-.13,1.77,;1.2,2.54,;-.12,.22,;-1.46,-.56,;-2.8,.22,;-4.14,-9.79,;-5.47,-9.02,;-4.14,-11.33,;5.18,-9.01,;6.52,-9.78,;5.18,-7.47,;7.85,-12.09,;9.18,-11.31,;6.52,-11.31,)| Show InChI InChI=1S/C34H32N3O18P/c38-16-2-5-19-25(12-16)55-26-13-17(39)3-6-20(26)29(19)18-4-1-15(11-21(18)31(44)45)30(43)36-22(32(46)47)7-9-27(40)35-24(34(50)51)14-54-56(52,53)37-23(33(48)49)8-10-28(41)42/h1-6,11-13,22-24,38H,7-10,14H2,(H,35,40)(H,36,43)(H,41,42)(H,44,45)(H,46,47)(H,48,49)(H,50,51)(H2,37,52,53)/p-1/t22-,23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA using N-[4-(phenylazo)-benzoyl]-glutamyl-gamma-glutamic acid as substrate after 15 mins by HPLC analysis |
Bioorg Med Chem Lett 21: 7013-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.115
BindingDB Entry DOI: 10.7270/Q2PG1S5S |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50331102
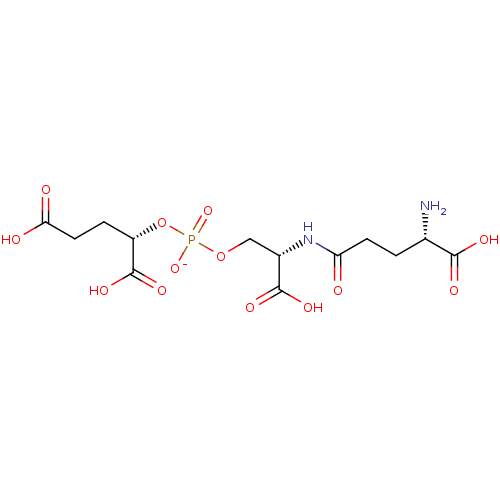
(2-{[2-(4-Amino-4-carboxy-butyrylamino)-2-carboxyet...)Show SMILES N[C@@H](CCC(=O)N[C@@H](COP([O-])(=O)O[C@@H](CCC(O)=O)C(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C13H21N2O13P/c14-6(11(19)20)1-3-9(16)15-7(12(21)22)5-27-29(25,26)28-8(13(23)24)2-4-10(17)18/h6-8H,1-5,14H2,(H,15,16)(H,17,18)(H,19,20)(H,21,22)(H,23,24)(H,25,26)/p-1/t6-,7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA in human LNCaP cells |
Bioorg Med Chem Lett 20: 7124-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.057
BindingDB Entry DOI: 10.7270/Q2KD1Z57 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50222546

(CHEMBL241779 | tetralithium (2S)-2-(phosphonatoami...)Show SMILES [O-]C(=O)CC[C@H](NP([O-])([O-])=O)C([O-])=O Show InChI InChI=1S/C5H10NO7P/c7-4(8)2-1-3(5(9)10)6-14(11,12)13/h3H,1-2H2,(H,7,8)(H,9,10)(H3,6,11,12,13)/p-4/t3-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA |
Bioorg Med Chem 15: 7434-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.028
BindingDB Entry DOI: 10.7270/Q2057FMF |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50358647
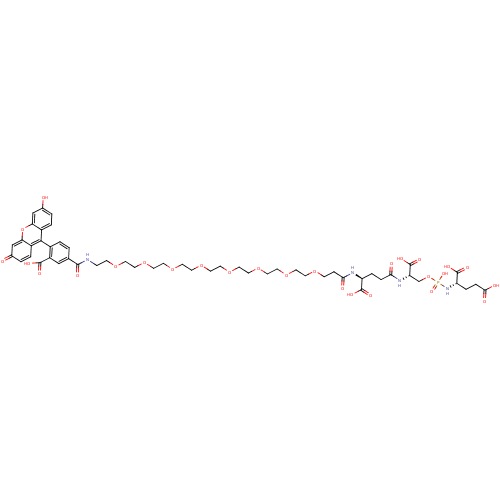
(CHEMBL1921900)Show SMILES OC(=O)CC[C@H](NP(O)(=O)OC[C@H](NC(=O)CC[C@H](NC(=O)CCOCCOCCOCCOCCOCCOCCOCCOCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(O)cc2oc2cc(=O)ccc12)C(O)=O)C(O)=O)C(O)=O |r,wD:18.18,12.12,5.5,(58.79,-21.53,;58.78,-20,;60.12,-19.23,;57.45,-19.23,;56.12,-20,;54.79,-19.23,;53.46,-20,;52.12,-19.23,;53.21,-18.14,;50.79,-20,;52.12,-17.69,;50.79,-16.92,;50.79,-15.39,;49.45,-14.61,;48.13,-15.38,;48.13,-16.92,;46.79,-14.61,;45.47,-15.38,;44.13,-14.61,;42.8,-15.38,;41.47,-14.61,;41.47,-13.08,;40.14,-15.38,;38.8,-14.61,;37.48,-15.38,;36.14,-14.62,;34.81,-15.39,;33.48,-14.62,;32.15,-15.39,;30.81,-14.62,;29.48,-15.39,;28.15,-14.62,;26.82,-15.39,;25.49,-14.62,;24.15,-15.39,;22.82,-14.62,;21.49,-15.39,;20.16,-14.62,;18.82,-15.39,;17.5,-14.62,;16.16,-15.39,;14.83,-14.63,;13.5,-15.39,;12.17,-14.63,;10.83,-15.4,;9.51,-14.63,;8.17,-15.4,;6.84,-14.63,;5.51,-15.4,;4.18,-14.63,;2.84,-15.4,;4.18,-13.09,;2.84,-12.32,;2.84,-10.78,;4.18,-10.02,;5.51,-10.78,;5.51,-12.31,;6.84,-10.01,;8.17,-10.77,;5.73,-8.91,;4.18,-8.47,;2.86,-7.71,;1.53,-8.48,;.19,-7.71,;.2,-6.17,;-1.14,-5.41,;1.52,-5.4,;2.85,-6.16,;4.19,-5.39,;5.53,-6.17,;6.85,-5.4,;8.19,-6.18,;9.52,-5.42,;8.18,-7.72,;6.84,-8.48,;5.52,-7.71,;44.13,-13.07,;45.47,-12.31,;42.8,-12.3,;52.12,-14.62,;53.46,-15.38,;52.12,-13.07,;54.79,-17.68,;56.12,-16.91,;53.45,-16.91,)| Show InChI InChI=1S/C53H69N4O27P/c58-34-2-5-37-43(30-34)84-44-31-35(59)3-6-38(44)48(37)36-4-1-33(29-39(36)50(65)66)49(64)54-12-14-76-16-18-78-20-22-80-24-26-82-28-27-81-25-23-79-21-19-77-17-15-75-13-11-46(61)55-40(51(67)68)7-9-45(60)56-42(53(71)72)32-83-85(73,74)57-41(52(69)70)8-10-47(62)63/h1-6,29-31,40-42,58H,7-28,32H2,(H,54,64)(H,55,61)(H,56,60)(H,62,63)(H,65,66)(H,67,68)(H,69,70)(H,71,72)(H2,57,73,74)/t40-,41-,42-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA using N-[4-(phenylazo)-benzoyl]-glutamyl-gamma-glutamic acid as substrate after 15 mins by HPLC analysis |
Bioorg Med Chem Lett 21: 7013-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.115
BindingDB Entry DOI: 10.7270/Q2PG1S5S |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50222549

((S)-2-phosphonoamino-pentanedioic acid | CHEMBL238...)Show InChI InChI=1S/C5H10NO7P/c7-4(8)2-1-3(5(9)10)6-14(11,12)13/h3H,1-2H2,(H,7,8)(H,9,10)(H3,6,11,12,13)/t3-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA |
Bioorg Med Chem 15: 7434-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.028
BindingDB Entry DOI: 10.7270/Q2057FMF |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50358644
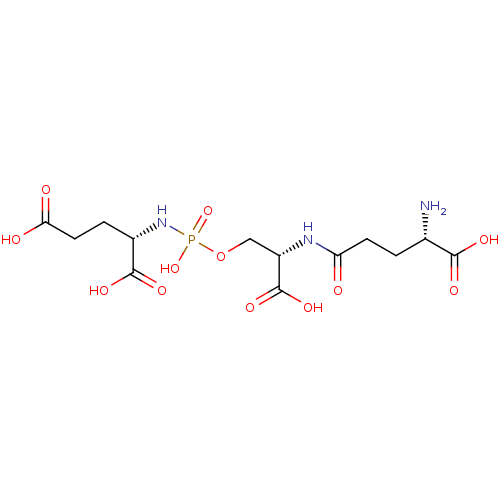
(CHEMBL1921897)Show SMILES N[C@@H](CCC(=O)N[C@@H](COP(O)(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C13H22N3O12P/c14-6(11(20)21)1-3-9(17)15-8(13(24)25)5-28-29(26,27)16-7(12(22)23)2-4-10(18)19/h6-8H,1-5,14H2,(H,15,17)(H,18,19)(H,20,21)(H,22,23)(H,24,25)(H2,16,26,27)/t6-,7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA using N-[4-(phenylazo)-benzoyl]-glutamyl-gamma-glutamic acid as substrate after 15 mins by HPLC analysis |
Bioorg Med Chem Lett 21: 7013-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.115
BindingDB Entry DOI: 10.7270/Q2PG1S5S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50222551

(CHEMBL241780 | tetralithium (2S)-2-(phosphonatooxy...)Show SMILES [O-]C(=O)CC[C@H](OP([O-])([O-])=O)C([O-])=O Show InChI InChI=1S/C5H9O8P/c6-4(7)2-1-3(5(8)9)13-14(10,11)12/h3H,1-2H2,(H,6,7)(H,8,9)(H2,10,11,12)/p-4/t3-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA |
Bioorg Med Chem 15: 7434-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.028
BindingDB Entry DOI: 10.7270/Q2057FMF |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50222550
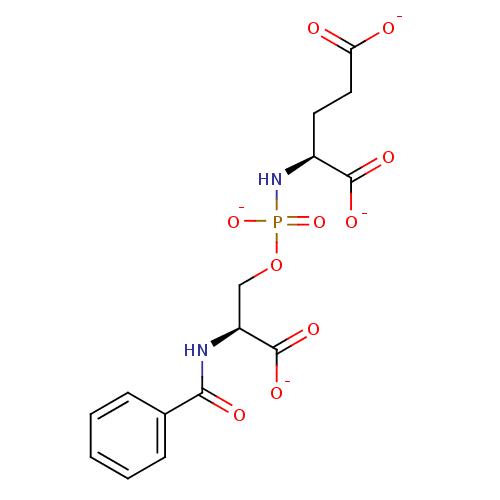
(CHEMBL239882 | tetrapotassium (3S,8S)-6-oxido-1-ox...)Show SMILES [O-]C(=O)CC[C@H](NP([O-])(=O)OC[C@H](NC(=O)c1ccccc1)C([O-])=O)C([O-])=O Show InChI InChI=1S/C15H19N2O10P/c18-12(19)7-6-10(14(21)22)17-28(25,26)27-8-11(15(23)24)16-13(20)9-4-2-1-3-5-9/h1-5,10-11H,6-8H2,(H,16,20)(H,18,19)(H,21,22)(H,23,24)(H2,17,25,26)/p-4/t10-,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA |
Bioorg Med Chem 15: 7434-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.028
BindingDB Entry DOI: 10.7270/Q2057FMF |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50222542

(CHEMBL238831 | tetrapotassium (3S,8S)-2-methyl-6-o...)Show SMILES CN([C@@H](COP([O-])(=O)N[C@@H](CCC([O-])=O)C([O-])=O)C([O-])=O)C(=O)c1ccccc1 Show InChI InChI=1S/C16H21N2O10P/c1-18(14(21)10-5-3-2-4-6-10)12(16(24)25)9-28-29(26,27)17-11(15(22)23)7-8-13(19)20/h2-6,11-12H,7-9H2,1H3,(H,19,20)(H,22,23)(H,24,25)(H2,17,26,27)/p-4/t11-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA |
Bioorg Med Chem 15: 7434-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.028
BindingDB Entry DOI: 10.7270/Q2057FMF |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50372201

(CHEMBL270610)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CCC4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OCCCOP([O-])(=O)N[C@@H](CCC([O-])=O)C([O-])=O Show InChI InChI=1S/C35H62NO8P/c1-23(2)8-6-9-24(3)28-12-13-29-27-11-10-25-22-26(16-18-34(25,4)30(27)17-19-35(28,29)5)43-20-7-21-44-45(41,42)36-31(33(39)40)14-15-32(37)38/h23-31H,6-22H2,1-5H3,(H,37,38)(H,39,40)(H2,36,41,42)/p-3/t24-,25?,26+,27+,28-,29+,30+,31+,34+,35-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of human PSMA |
Bioorg Med Chem Lett 18: 281-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.096
BindingDB Entry DOI: 10.7270/Q2N29XS3 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50372204
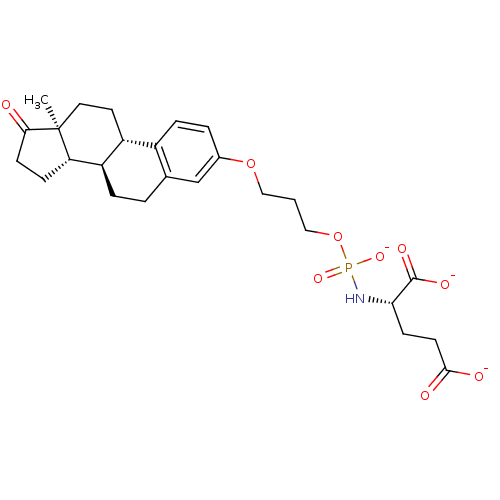
(CHEMBL402223)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OCCCOP([O-])(=O)N[C@@H](CCC([O-])=O)C([O-])=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C26H36NO9P/c1-26-12-11-19-18-6-4-17(15-16(18)3-5-20(19)21(26)7-9-23(26)28)35-13-2-14-36-37(33,34)27-22(25(31)32)8-10-24(29)30/h4,6,15,19-22H,2-3,5,7-14H2,1H3,(H,29,30)(H,31,32)(H2,27,33,34)/p-3/t19-,20-,21+,22+,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of human PSMA |
Bioorg Med Chem Lett 18: 281-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.096
BindingDB Entry DOI: 10.7270/Q2N29XS3 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50331103

((S)-2-carboxy-2-((S)-4-carboxy-4-(6-(2-(5-(3-ethyl...)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CCCCCC(=O)N[C@@H](CCC(=O)N[C@@H](COP([O-])(=O)O[C@@H](CCC(O)=O)C(O)=O)C(O)=O)C(O)=O)c3ccc4c(cc(cc4c3C2(C)C)S(O)(=O)=O)S(O)(=O)=O)C(C)(C)c2c1ccc1c(cc(cc21)S(O)(=O)=O)S(O)(=O)=O |r,c:9| Show InChI InChI=1S/C54H63N4O26PS4/c1-6-57-38-19-16-32-34(25-30(86(71,72)73)27-41(32)88(77,78)79)48(38)53(2,3)43(57)13-9-7-10-14-44-54(4,5)49-35-26-31(87(74,75)76)28-42(89(80,81)82)33(35)17-20-39(49)58(44)24-12-8-11-15-45(59)55-36(50(63)64)18-22-46(60)56-37(51(65)66)29-83-85(69,70)84-40(52(67)68)21-23-47(61)62/h7,9-10,13-14,16-17,19-20,25-28,36-37,40H,6,8,11-12,15,18,21-24,29H2,1-5H3,(H10-,55,56,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82)/t36-,37-,40-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA in human LNCaP cells |
Bioorg Med Chem Lett 20: 7124-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.057
BindingDB Entry DOI: 10.7270/Q2KD1Z57 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50222545

(CHEMBL394011 | tripotassium (2S)-2-{[(4-phenylbuto...)Show SMILES [O-]C(=O)CC[C@H](NP([O-])(=O)OCCCCc1ccccc1)C([O-])=O Show InChI InChI=1S/C15H22NO7P/c17-14(18)10-9-13(15(19)20)16-24(21,22)23-11-5-4-8-12-6-2-1-3-7-12/h1-3,6-7,13H,4-5,8-11H2,(H,17,18)(H,19,20)(H2,16,21,22)/p-3/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of human PSMA |
Bioorg Med Chem Lett 18: 281-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.096
BindingDB Entry DOI: 10.7270/Q2N29XS3 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50222545

(CHEMBL394011 | tripotassium (2S)-2-{[(4-phenylbuto...)Show SMILES [O-]C(=O)CC[C@H](NP([O-])(=O)OCCCCc1ccccc1)C([O-])=O Show InChI InChI=1S/C15H22NO7P/c17-14(18)10-9-13(15(19)20)16-24(21,22)23-11-5-4-8-12-6-2-1-3-7-12/h1-3,6-7,13H,4-5,8-11H2,(H,17,18)(H,19,20)(H2,16,21,22)/p-3/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA |
Bioorg Med Chem 15: 7434-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.028
BindingDB Entry DOI: 10.7270/Q2057FMF |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50222552

(CHEMBL239041 | tripotassium (2S)-2-[({2-[benzoyl(m...)Show SMILES CN(CCOP([O-])(=O)N[C@@H](CCC([O-])=O)C([O-])=O)C(=O)c1ccccc1 Show InChI InChI=1S/C15H21N2O8P/c1-17(14(20)11-5-3-2-4-6-11)9-10-25-26(23,24)16-12(15(21)22)7-8-13(18)19/h2-6,12H,7-10H2,1H3,(H,18,19)(H,21,22)(H2,16,23,24)/p-3/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA |
Bioorg Med Chem 15: 7434-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.028
BindingDB Entry DOI: 10.7270/Q2057FMF |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50088185
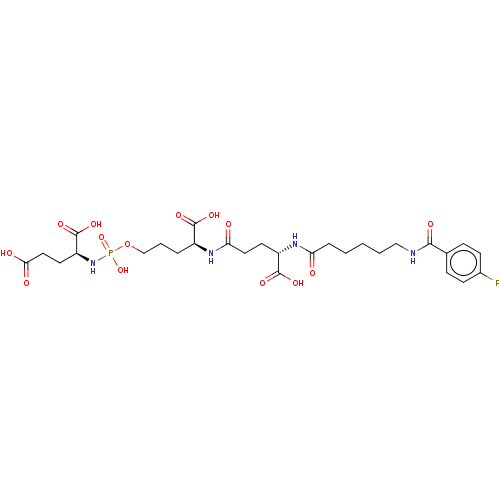
(CHEMBL3427435)Show SMILES OC(=O)CC[C@H](NP(O)(=O)OCCC[C@H](NC(=O)CC[C@H](NC(=O)CCCCCNC(=O)c1ccc(F)cc1)C(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C28H40FN4O14P/c29-18-9-7-17(8-10-18)25(38)30-15-3-1-2-6-22(34)32-20(27(41)42)11-13-23(35)31-19(26(39)40)5-4-16-47-48(45,46)33-21(28(43)44)12-14-24(36)37/h7-10,19-21H,1-6,11-16H2,(H,30,38)(H,31,35)(H,32,34)(H,36,37)(H,39,40)(H,41,42)(H,43,44)(H2,33,45,46)/t19-,20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method |
Bioorg Med Chem Lett 25: 2536-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.047
BindingDB Entry DOI: 10.7270/Q29G5PJK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50372203

(CHEMBL270594)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CCC4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)OP([O-])(=O)N[C@@H](CCC([O-])=O)C([O-])=O Show InChI InChI=1S/C32H56NO7P/c1-20(2)7-6-8-21(3)25-11-12-26-24-10-9-22-19-23(15-17-31(22,4)27(24)16-18-32(25,26)5)40-41(38,39)33-28(30(36)37)13-14-29(34)35/h20-28H,6-19H2,1-5H3,(H,34,35)(H,36,37)(H2,33,38,39)/p-3/t21-,22?,23+,24+,25-,26+,27+,28+,31+,32-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 435 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of human PSMA |
Bioorg Med Chem Lett 18: 281-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.096
BindingDB Entry DOI: 10.7270/Q2N29XS3 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50372205

(CHEMBL270824)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@H]4C[C@H](CC[C@]34C)OCCCOP([O-])(=O)N[C@@H](CCC([O-])=O)C([O-])=O)[C@@H]1CCC2=O Show InChI InChI=1S/C27H44NO9P/c1-26-12-10-18(36-14-3-15-37-38(34,35)28-22(25(32)33)7-9-24(30)31)16-17(26)4-5-19-20-6-8-23(29)27(20,2)13-11-21(19)26/h17-22H,3-16H2,1-2H3,(H,30,31)(H,32,33)(H2,28,34,35)/p-3/t17-,18-,19-,20-,21-,22-,26-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 438 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of human PSMA |
Bioorg Med Chem Lett 18: 281-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.096
BindingDB Entry DOI: 10.7270/Q2N29XS3 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50372202
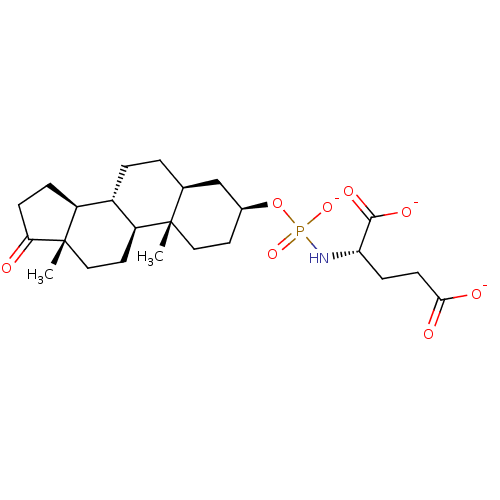
(CHEMBL437209)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@H]4C[C@H](CC[C@]34C)OP([O-])(=O)N[C@@H](CCC([O-])=O)C([O-])=O)[C@@H]1CCC2=O Show InChI InChI=1S/C24H38NO8P/c1-23-11-9-15(33-34(31,32)25-19(22(29)30)6-8-21(27)28)13-14(23)3-4-16-17-5-7-20(26)24(17,2)12-10-18(16)23/h14-19H,3-13H2,1-2H3,(H,27,28)(H,29,30)(H2,25,31,32)/p-3/t14-,15-,16-,17-,18-,19-,23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 569 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of human PSMA |
Bioorg Med Chem Lett 18: 281-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.096
BindingDB Entry DOI: 10.7270/Q2N29XS3 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50372206

(CHEMBL402033)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OC(=O)c5ccccc5)ccc34)[C@@H]1CC[C@@H]2OCCCOP([O-])(=O)N[C@@H](CCC([O-])=O)C([O-])=O Show InChI InChI=1S/C33H42NO10P/c1-33-17-16-25-24-11-9-23(44-32(39)21-6-3-2-4-7-21)20-22(24)8-10-26(25)27(33)12-14-29(33)42-18-5-19-43-45(40,41)34-28(31(37)38)13-15-30(35)36/h2-4,6-7,9,11,20,25-29H,5,8,10,12-19H2,1H3,(H,35,36)(H,37,38)(H2,34,40,41)/p-3/t25-,26-,27+,28+,29+,33+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 572 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of human PSMA |
Bioorg Med Chem Lett 18: 281-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.096
BindingDB Entry DOI: 10.7270/Q2N29XS3 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50212958

(4-(4-(3,4-dihydroxyphenyl)butyl)benzene-1,2-diol |...)Show InChI InChI=1S/C16H18O4/c17-13-7-5-11(9-15(13)19)3-1-2-4-12-6-8-14(18)16(20)10-12/h5-10,17-20H,1-4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50222543

(CHEMBL239465 | tetrapotassium (3R,8S)-6-oxido-1-ox...)Show SMILES [O-]C(=O)CC[C@H](NP([O-])(=O)OC[C@@H](NC(=O)c1ccccc1)C([O-])=O)C([O-])=O Show InChI InChI=1S/C15H19N2O10P/c18-12(19)7-6-10(14(21)22)17-28(25,26)27-8-11(15(23)24)16-13(20)9-4-2-1-3-5-9/h1-5,10-11H,6-8H2,(H,16,20)(H,18,19)(H,21,22)(H,23,24)(H2,17,25,26)/p-4/t10-,11+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA |
Bioorg Med Chem 15: 7434-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.028
BindingDB Entry DOI: 10.7270/Q2057FMF |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50222548
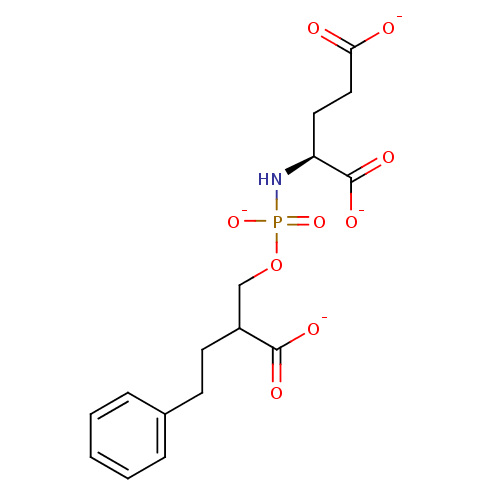
(CHEMBL391840 | tetrapotassium (2S)-2-{[(2-carboxyl...)Show SMILES [O-]C(=O)CC[C@H](NP([O-])(=O)OCC(CCc1ccccc1)C([O-])=O)C([O-])=O Show InChI InChI=1S/C16H22NO9P/c18-14(19)9-8-13(16(22)23)17-27(24,25)26-10-12(15(20)21)7-6-11-4-2-1-3-5-11/h1-5,12-13H,6-10H2,(H,18,19)(H,20,21)(H,22,23)(H2,17,24,25)/p-4/t12?,13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA |
Bioorg Med Chem 15: 7434-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.028
BindingDB Entry DOI: 10.7270/Q2057FMF |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50212959

(4-(4-p-tolylbutyl)benzene-1,2-diol | CHEMBL245942)Show InChI InChI=1S/C17H20O2/c1-13-6-8-14(9-7-13)4-2-3-5-15-10-11-16(18)17(19)12-15/h6-12,18-19H,2-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50212960

(4-(4-(4-chlorophenyl)butyl)benzene-1,2-diol | CHEM...)Show InChI InChI=1S/C16H17ClO2/c17-14-8-5-12(6-9-14)3-1-2-4-13-7-10-15(18)16(19)11-13/h5-11,18-19H,1-4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50212956
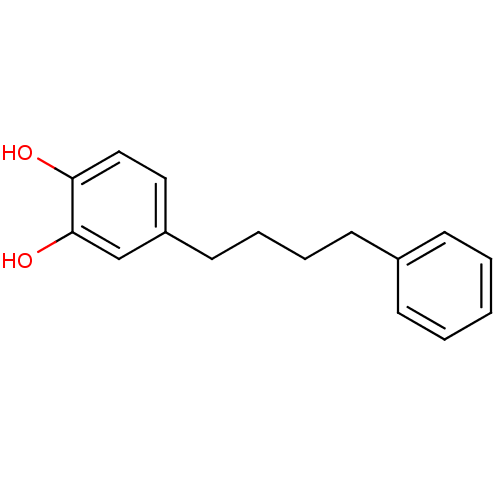
(4-(4-phenylbutyl)benzene-1,2-diol | CHEMBL442420)Show InChI InChI=1S/C16H18O2/c17-15-11-10-14(12-16(15)18)9-5-4-8-13-6-2-1-3-7-13/h1-3,6-7,10-12,17-18H,4-5,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM22372
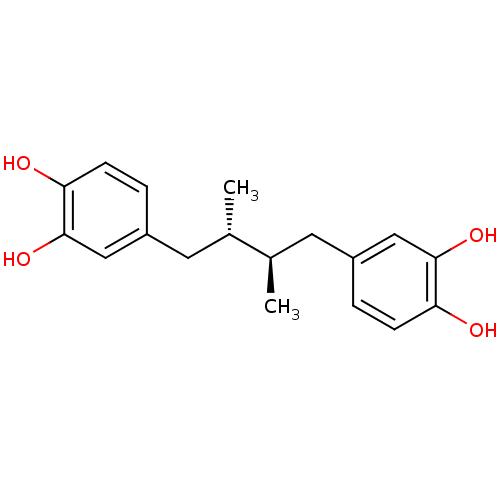
(4-[(2R,3S)-4-(3,4-dihydroxyphenyl)-2,3-dimethylbut...)Show SMILES C[C@@H](Cc1ccc(O)c(O)c1)[C@H](C)Cc1ccc(O)c(O)c1 Show InChI InChI=1S/C18H22O4/c1-11(7-13-3-5-15(19)17(21)9-13)12(2)8-14-4-6-16(20)18(22)10-14/h3-6,9-12,19-22H,7-8H2,1-2H3/t11-,12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50212961

(5-phenyl-5,6,7,8-tetrahydronaphthalene-2,3-diol | ...)Show InChI InChI=1S/C16H16O2/c17-15-9-12-7-4-8-13(14(12)10-16(15)18)11-5-2-1-3-6-11/h1-3,5-6,9-10,13,17-18H,4,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50212955

(4-(4-(4-hydroxyphenyl)butyl)benzene-1,2-diol | CHE...)Show InChI InChI=1S/C16H18O3/c17-14-8-5-12(6-9-14)3-1-2-4-13-7-10-15(18)16(19)11-13/h5-11,17-19H,1-4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50212957

(4-(4-(3,4-dichlorophenyl)butyl)benzene-1,2-diol | ...)Show InChI InChI=1S/C16H16Cl2O2/c17-13-7-5-11(9-14(13)18)3-1-2-4-12-6-8-15(19)16(20)10-12/h5-10,19-20H,1-4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50212962
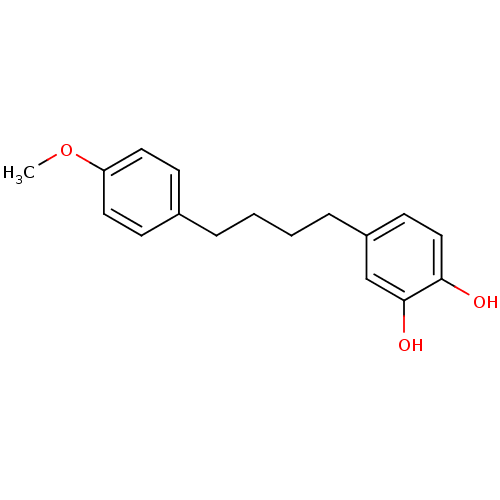
(4-(4-(4-methoxyphenyl)butyl)benzene-1,2-diol | CHE...)Show InChI InChI=1S/C17H20O3/c1-20-15-9-6-13(7-10-15)4-2-3-5-14-8-11-16(18)17(19)12-14/h6-12,18-19H,2-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50088192
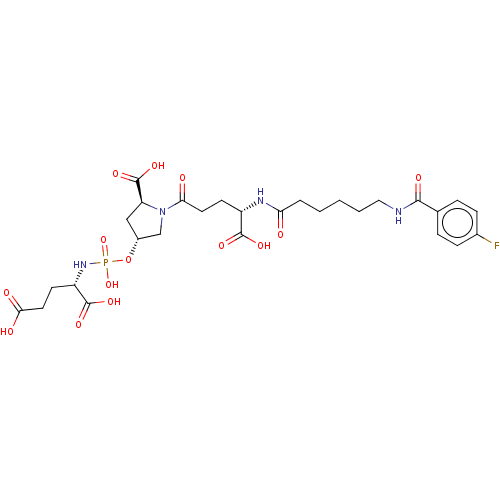
(CHEMBL3427442)Show SMILES OC(=O)CC[C@H](NP(O)(=O)O[C@@H]1C[C@H](N(C1)C(=O)CC[C@H](NC(=O)CCCCCNC(=O)c1ccc(F)cc1)C(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C28H38FN4O14P/c29-17-7-5-16(6-8-17)25(38)30-13-3-1-2-4-22(34)31-19(26(39)40)9-11-23(35)33-15-18(14-21(33)28(43)44)47-48(45,46)32-20(27(41)42)10-12-24(36)37/h5-8,18-21H,1-4,9-15H2,(H,30,38)(H,31,34)(H,36,37)(H,39,40)(H,41,42)(H,43,44)(H2,32,45,46)/t18-,19+,20+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method |
Bioorg Med Chem Lett 25: 2536-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.047
BindingDB Entry DOI: 10.7270/Q29G5PJK |
More data for this
Ligand-Target Pair | |
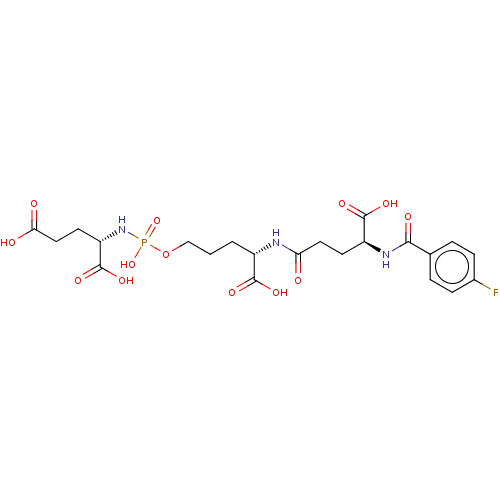
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50088182
(CHEMBL3427434)Show SMILES OC(=O)CC[C@H](NP(O)(=O)OCCC[C@H](NC(=O)CC[C@H](NC(=O)c1ccc(F)cc1)C(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C22H29FN3O13P/c23-13-5-3-12(4-6-13)19(30)25-15(21(33)34)7-9-17(27)24-14(20(31)32)2-1-11-39-40(37,38)26-16(22(35)36)8-10-18(28)29/h3-6,14-16H,1-2,7-11H2,(H,24,27)(H,25,30)(H,28,29)(H,31,32)(H,33,34)(H,35,36)(H2,26,37,38)/t14-,15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method |
Bioorg Med Chem Lett 25: 2536-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.047
BindingDB Entry DOI: 10.7270/Q29G5PJK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50222547
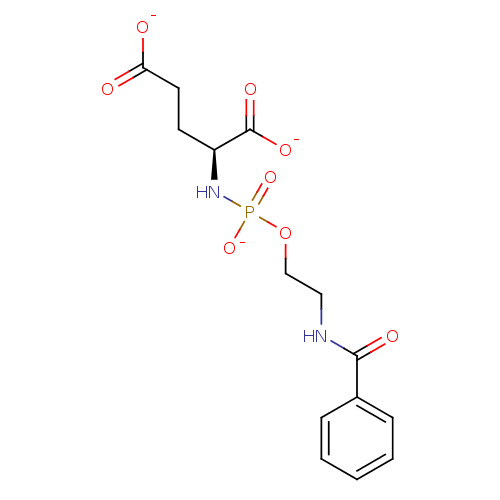
(CHEMBL400842 | tripotassium (2S)-2-({[2-(benzoylam...)Show SMILES [O-]C(=O)CC[C@H](NP([O-])(=O)OCCNC(=O)c1ccccc1)C([O-])=O Show InChI InChI=1S/C14H19N2O8P/c17-12(18)7-6-11(14(20)21)16-25(22,23)24-9-8-15-13(19)10-4-2-1-3-5-10/h1-5,11H,6-9H2,(H,15,19)(H,17,18)(H,20,21)(H2,16,22,23)/p-3/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA |
Bioorg Med Chem 15: 7434-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.028
BindingDB Entry DOI: 10.7270/Q2057FMF |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50088190

(CHEMBL3427440)Show SMILES OC(=O)CC[C@H](NP(O)(=O)O[C@@H]1C[C@H](N(C1)C(=O)CC[C@H](NC(=O)c1ccc(F)cc1)C(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C22H27FN3O13P/c23-12-3-1-11(2-4-12)19(30)24-14(20(31)32)5-7-17(27)26-10-13(9-16(26)22(35)36)39-40(37,38)25-15(21(33)34)6-8-18(28)29/h1-4,13-16H,5-10H2,(H,24,30)(H,28,29)(H,31,32)(H,33,34)(H,35,36)(H2,25,37,38)/t13-,14+,15+,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method |
Bioorg Med Chem Lett 25: 2536-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.047
BindingDB Entry DOI: 10.7270/Q29G5PJK |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50088191
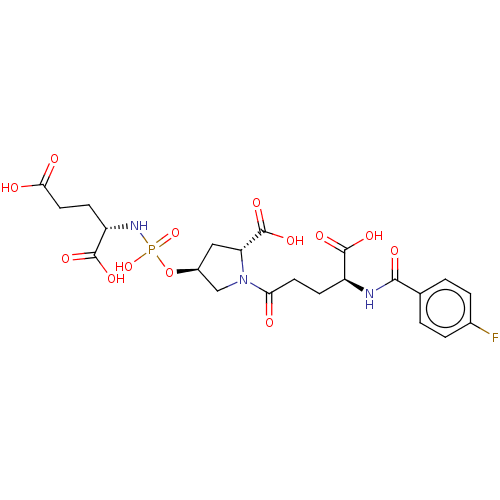
(CHEMBL3427441)Show SMILES OC(=O)CC[C@H](NP(O)(=O)O[C@H]1C[C@@H](N(C1)C(=O)CC[C@H](NC(=O)c1ccc(F)cc1)C(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C22H27FN3O13P/c23-12-3-1-11(2-4-12)19(30)24-14(20(31)32)5-7-17(27)26-10-13(9-16(26)22(35)36)39-40(37,38)25-15(21(33)34)6-8-18(28)29/h1-4,13-16H,5-10H2,(H,24,30)(H,28,29)(H,31,32)(H,33,34)(H,35,36)(H2,25,37,38)/t13-,14-,15-,16+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method |
Bioorg Med Chem Lett 25: 2536-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.047
BindingDB Entry DOI: 10.7270/Q29G5PJK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM22372

(4-[(2R,3S)-4-(3,4-dihydroxyphenyl)-2,3-dimethylbut...)Show SMILES C[C@@H](Cc1ccc(O)c(O)c1)[C@H](C)Cc1ccc(O)c(O)c1 Show InChI InChI=1S/C18H22O4/c1-11(7-13-3-5-15(19)17(21)9-13)12(2)8-14-4-6-16(20)18(22)10-14/h3-6,9-12,19-22H,7-8H2,1-2H3/t11-,12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of 15 LOX |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50212958

(4-(4-(3,4-dihydroxyphenyl)butyl)benzene-1,2-diol |...)Show InChI InChI=1S/C16H18O4/c17-13-7-5-11(9-15(13)19)3-1-2-4-12-6-8-14(18)16(20)10-12/h5-10,17-20H,1-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of 15 LOX |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50222544

(CHEMBL391138 | tetrapotassium (2S)-2-{[(3-oxido-3-...)Show SMILES [O-]C(=O)CCOP([O-])(=O)N[C@@H](CCC([O-])=O)C([O-])=O Show InChI InChI=1S/C8H14NO9P/c10-6(11)2-1-5(8(14)15)9-19(16,17)18-4-3-7(12)13/h5H,1-4H2,(H,10,11)(H,12,13)(H,14,15)(H2,9,16,17)/p-4/t5-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA |
Bioorg Med Chem 15: 7434-43 (2007)
Article DOI: 10.1016/j.bmc.2007.07.028
BindingDB Entry DOI: 10.7270/Q2057FMF |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50331104

(6-(2-(-5-(3-ethyl-1,1-dimethyl-6,8-disulfo-1H-benz...)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CCCCCC([O-])=O)c3ccc4c(cc(cc4c3C2(C)C)S(O)(=O)=O)S(O)(=O)=O)C(C)(C)c2c1ccc1c(cc(cc21)S(O)(=O)=O)S(O)(=O)=O |c:9| Show InChI InChI=1S/C41H44N2O14S4/c1-6-42-31-18-16-27-29(21-25(58(46,47)48)23-33(27)60(52,53)54)38(31)40(2,3)35(42)13-9-7-10-14-36-41(4,5)39-30-22-26(59(49,50)51)24-34(61(55,56)57)28(30)17-19-32(39)43(36)20-12-8-11-15-37(44)45/h7,9-10,13-14,16-19,21-24H,6,8,11-12,15,20H2,1-5H3,(H4-,44,45,46,47,48,49,50,51,52,53,54,55,56,57) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA in human LNCaP cells |
Bioorg Med Chem Lett 20: 7124-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.057
BindingDB Entry DOI: 10.7270/Q2KD1Z57 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50212956

(4-(4-phenylbutyl)benzene-1,2-diol | CHEMBL442420)Show InChI InChI=1S/C16H18O2/c17-15-11-10-14(12-16(15)18)9-5-4-8-13-6-2-1-3-7-13/h1-3,6-7,10-12,17-18H,4-5,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of 15 LOX |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50212955

(4-(4-(4-hydroxyphenyl)butyl)benzene-1,2-diol | CHE...)Show InChI InChI=1S/C16H18O3/c17-14-8-5-12(6-9-14)3-1-2-4-13-7-10-15(18)16(19)11-13/h5-11,17-19H,1-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of 15 LOX |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50088193
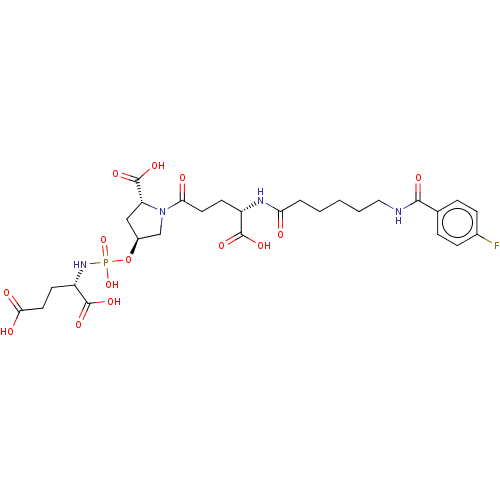
(CHEMBL3427443)Show SMILES OC(=O)CC[C@H](NP(O)(=O)O[C@H]1C[C@@H](N(C1)C(=O)CC[C@H](NC(=O)CCCCCNC(=O)c1ccc(F)cc1)C(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C28H38FN4O14P/c29-17-7-5-16(6-8-17)25(38)30-13-3-1-2-4-22(34)31-19(26(39)40)9-11-23(35)33-15-18(14-21(33)28(43)44)47-48(45,46)32-20(27(41)42)10-12-24(36)37/h5-8,18-21H,1-4,9-15H2,(H,30,38)(H,31,34)(H,36,37)(H,39,40)(H,41,42)(H,43,44)(H2,32,45,46)/t18-,19-,20-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method |
Bioorg Med Chem Lett 25: 2536-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.047
BindingDB Entry DOI: 10.7270/Q29G5PJK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50212962

(4-(4-(4-methoxyphenyl)butyl)benzene-1,2-diol | CHE...)Show InChI InChI=1S/C17H20O3/c1-20-15-9-6-13(7-10-15)4-2-3-5-14-8-11-16(18)17(19)12-14/h6-12,18-19H,2-5H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of 15 LOX |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50212960

(4-(4-(4-chlorophenyl)butyl)benzene-1,2-diol | CHEM...)Show InChI InChI=1S/C16H17ClO2/c17-14-8-5-12(6-9-14)3-1-2-4-13-7-10-15(18)16(19)11-13/h5-11,18-19H,1-4H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of 15 LOX |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50088183
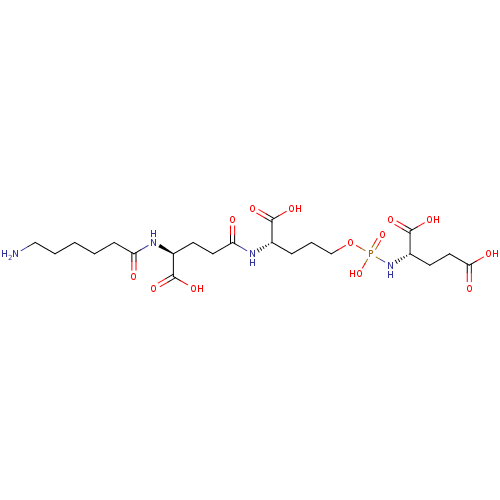
(CHEMBL3427433)Show SMILES NCCCCCC(=O)N[C@@H](CCC(=O)N[C@@H](CCCOP(O)(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H37N4O13P/c22-11-3-1-2-6-16(26)24-14(20(32)33)7-9-17(27)23-13(19(30)31)5-4-12-38-39(36,37)25-15(21(34)35)8-10-18(28)29/h13-15H,1-12,22H2,(H,23,27)(H,24,26)(H,28,29)(H,30,31)(H,32,33)(H,34,35)(H2,25,36,37)/t13-,14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method |
Bioorg Med Chem Lett 25: 2536-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.047
BindingDB Entry DOI: 10.7270/Q29G5PJK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50212959

(4-(4-p-tolylbutyl)benzene-1,2-diol | CHEMBL245942)Show InChI InChI=1S/C17H20O2/c1-13-6-8-14(9-7-13)4-2-3-5-15-10-11-16(18)17(19)12-15/h6-12,18-19H,2-5H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
San Francisco State University
Curated by ChEMBL
| Assay Description
Inhibition of 15 LOX |
Bioorg Med Chem Lett 17: 4026-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.092
BindingDB Entry DOI: 10.7270/Q20Z743X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data