

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50017292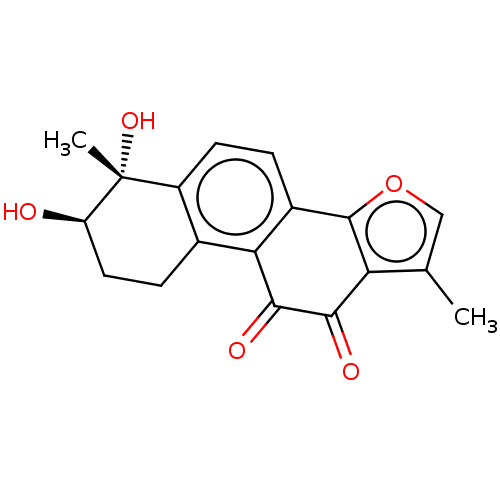 (CHEMBL3287734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Competitive inhibition of EZH2 histone methyltransferase activity in EZH2/SUZ12/EED/RbAp46/48 (unknown origin) using histone H3 peptide/varying conce... | Bioorg Med Chem Lett 24: 2486-92 (2014) Article DOI: 10.1016/j.bmcl.2014.04.010 BindingDB Entry DOI: 10.7270/Q2BR8TR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210] (Homo sapiens (Human)) | BDBM538119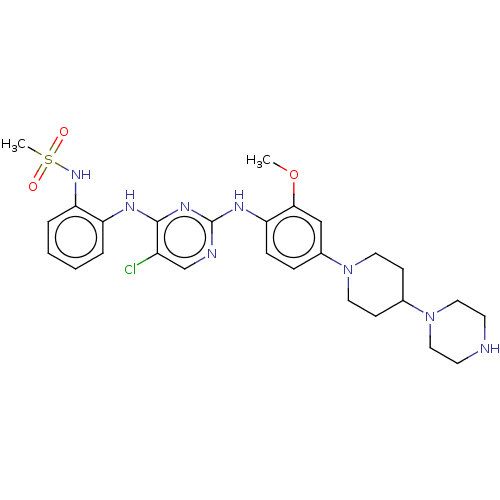 (US11253516, Example 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210] (Homo sapiens (Human)) | BDBM538123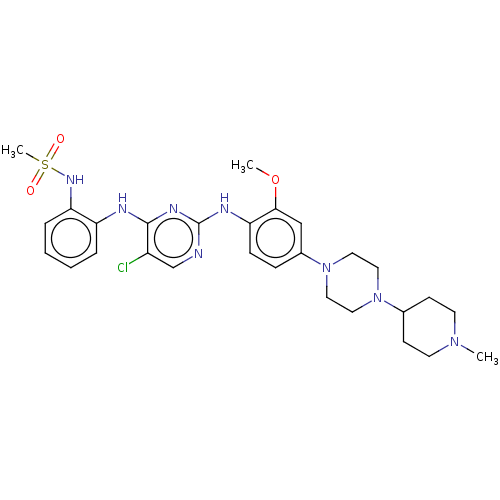 (US11253516, Example 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538108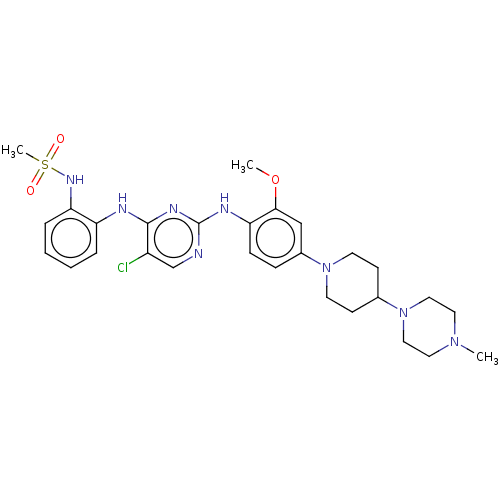 (US11253516, Example 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538135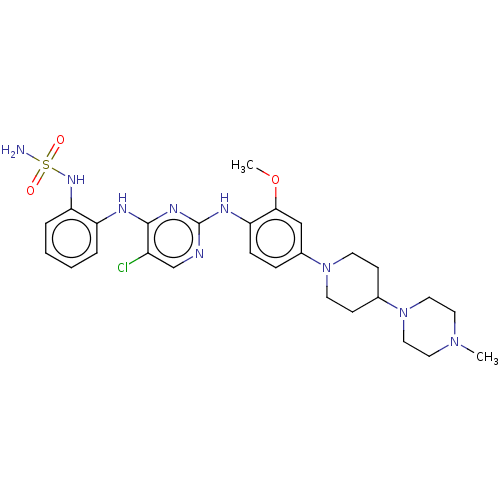 (US11253516, Example 37) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538139 (US11253516, Example 41) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50384024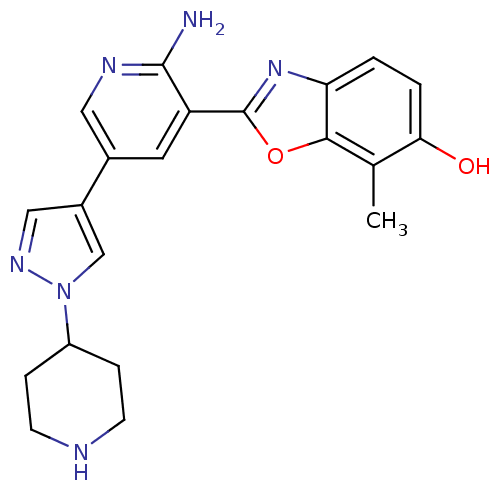 (CHEMBL2032280 | CHEMBL2079349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met by time resolved-fluorescence resonance energy transfer analysis | Bioorg Med Chem Lett 22: 4044-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.083 BindingDB Entry DOI: 10.7270/Q2FF3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243386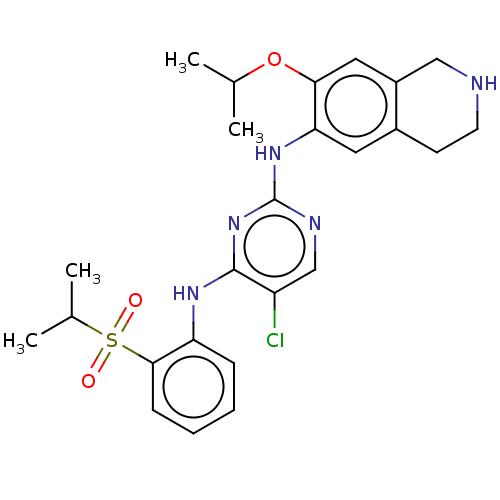 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(7-is...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243402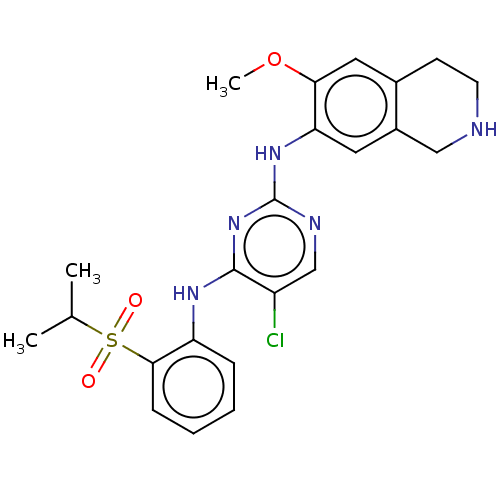 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(6-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of crizotinib-resistant ALK G1269A mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc... | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243393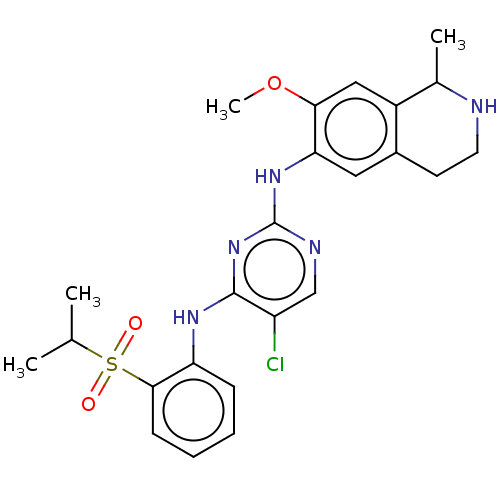 (5-chloro-N4-2-(isopropylsulfonyl)phenyl)-N2-(7-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538140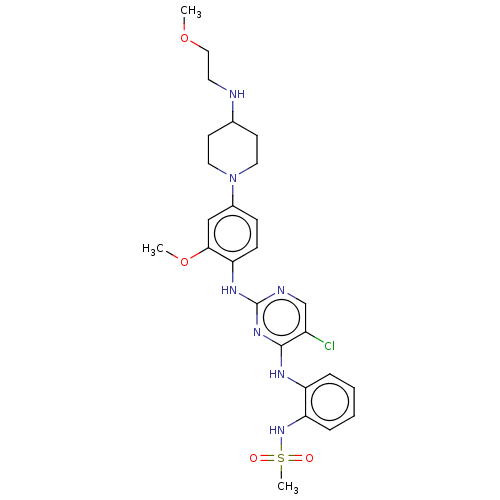 (US11253516, Example 42) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538137 (US11253516, Example 39) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538134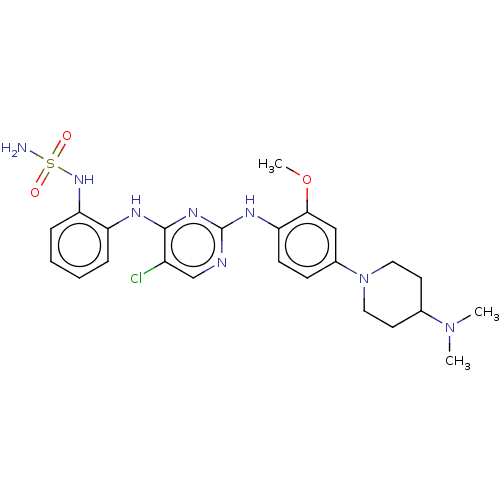 (US11253516, Example 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50380974 (CHEMBL2016903) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant VEGFR-2 kinase domain by homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 2837-42 (2012) Article DOI: 10.1016/j.bmcl.2012.02.073 BindingDB Entry DOI: 10.7270/Q2RN38W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243395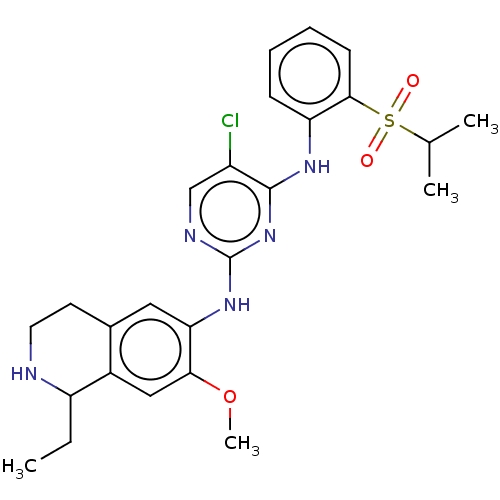 (5-chloro-N4-2-(isopropylsulfonyl)phenyl)-N2-(7-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM243393 (5-chloro-N4-2-(isopropylsulfonyl)phenyl)-N2-(7-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210] (Homo sapiens (Human)) | BDBM538125 (US11253516, Example 27) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50116683 (CHEMBL3608526 | US10053458, 49) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210] (Homo sapiens (Human)) | BDBM538120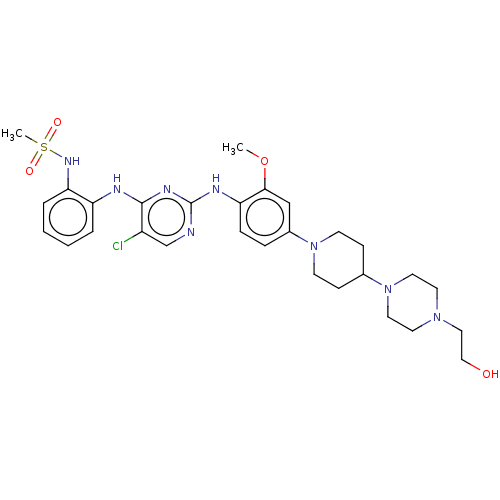 (US11253516, Example 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538138 (US11253516, Example 40) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM291934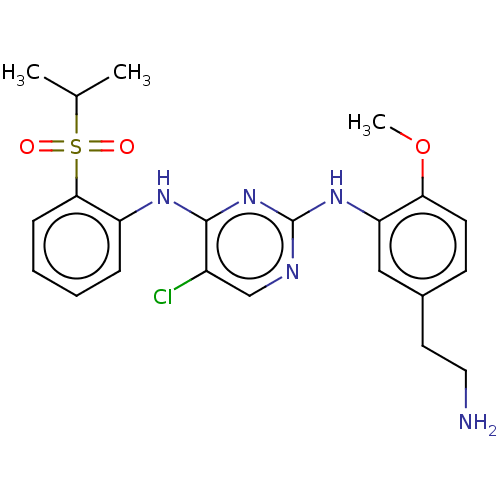 (US10100019, Example 4) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | 25 |
Korea Research Institute of Chemical Technology US Patent | Assay Description The following experiment was performed in order to measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of ... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243402 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(6-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of crizotinib-resistant ALK C1156Y mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc... | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243402 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(6-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of crizotinib-resistant ALK F1174L mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc... | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538136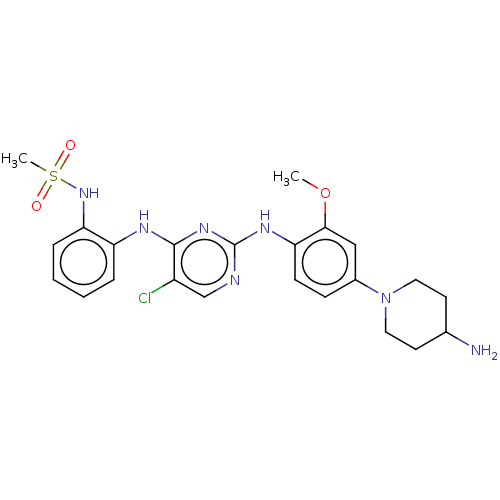 (US11253516, Example 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50380971 (CHEMBL2016927) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant VEGFR-2 kinase domain by homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 2837-42 (2012) Article DOI: 10.1016/j.bmcl.2012.02.073 BindingDB Entry DOI: 10.7270/Q2RN38W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50116690 (CHEMBL3608528 | US10053458, 67) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243386 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(7-is...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of ALK (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescence assay | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50116696 (CHEMBL3608534 | US10053458, 52) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50384038 (CHEMBL2032155) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant FLT3 by time resolved-fluorescence resonance energy transfer analysis | Bioorg Med Chem Lett 22: 4044-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.083 BindingDB Entry DOI: 10.7270/Q2FF3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538141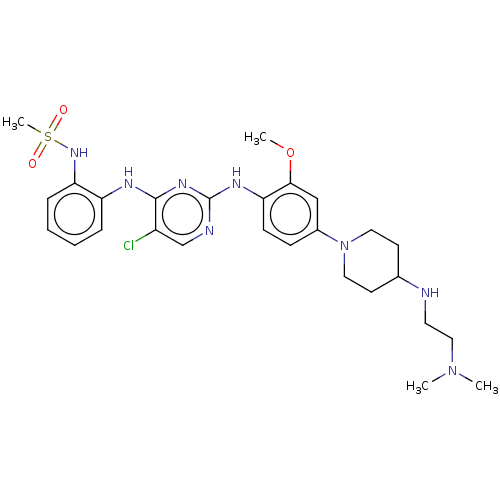 (US11253516, Example 43) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50116699 (CHEMBL3608642 | US10053458, 53) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210] (Homo sapiens (Human)) | BDBM538108 (US11253516, Example 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM50116696 (CHEMBL3608534 | US10053458, 52) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210] (Homo sapiens (Human)) | BDBM538117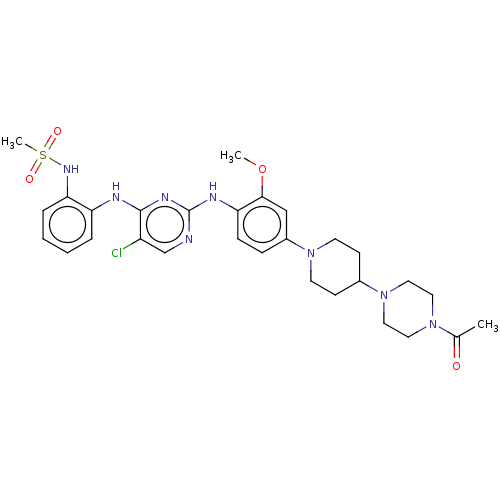 (US11253516, Example 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50380968 (CHEMBL2016924) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant VEGFR-2 kinase domain by homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 2837-42 (2012) Article DOI: 10.1016/j.bmcl.2012.02.073 BindingDB Entry DOI: 10.7270/Q2RN38W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50116685 (CHEMBL3608644 | US10053458, 54) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538120 (US11253516, Example 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM291943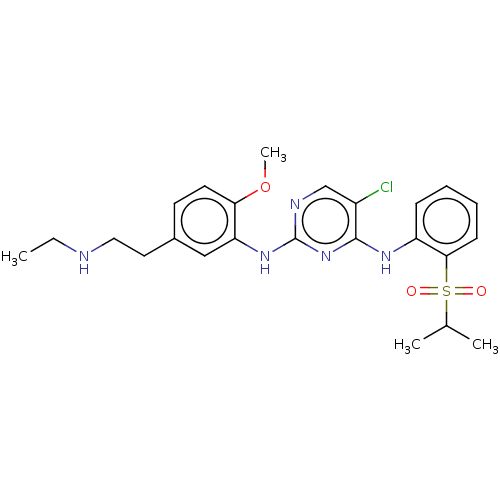 (US10100019, Example 13) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | 25 |
Korea Research Institute of Chemical Technology US Patent | Assay Description The following experiment was performed in order to measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of ... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM291934 (US10100019, Example 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology US Patent | Assay Description To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM291954 (US10100019, Example 24) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | 25 |
Korea Research Institute of Chemical Technology US Patent | Assay Description The following experiment was performed in order to measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of ... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210] (Homo sapiens (Human)) | BDBM538126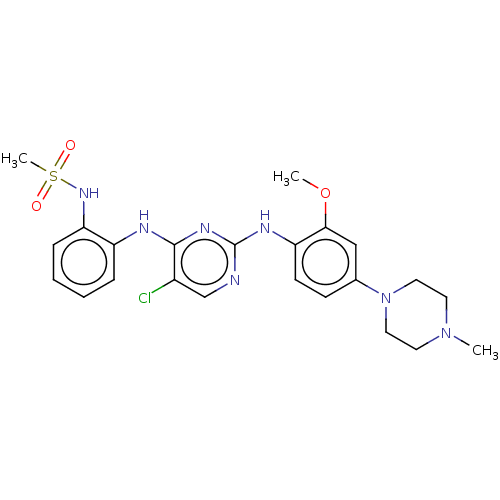 (US11253516, Example 28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM50116680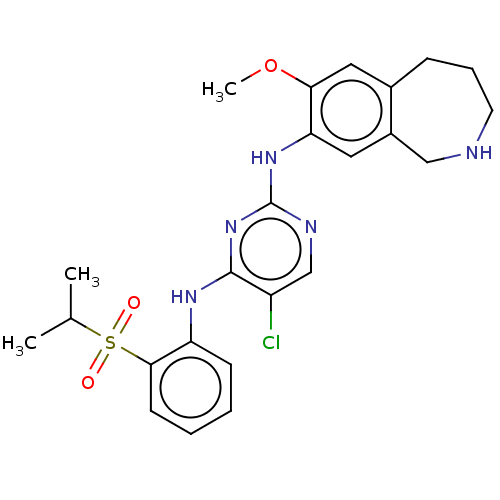 (CHEMBL3608523 | US10053458, 56) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243431 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(6-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM50116699 (CHEMBL3608642 | US10053458, 53) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM291951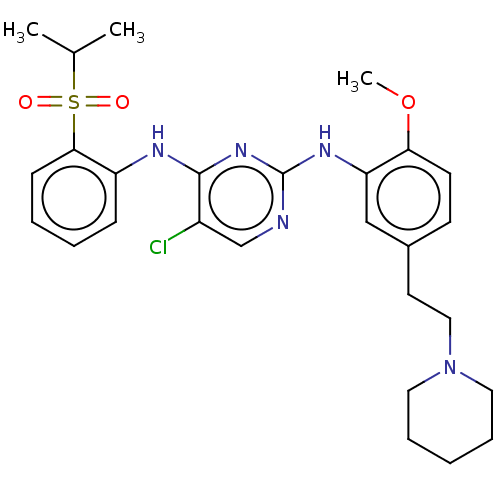 (US10100019, Example 21) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Korea Research Institute of Chemical Technology US Patent | Assay Description The following experiment was performed in order to measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of ... | US Patent US10100019 (2018) BindingDB Entry DOI: 10.7270/Q2125VPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50116692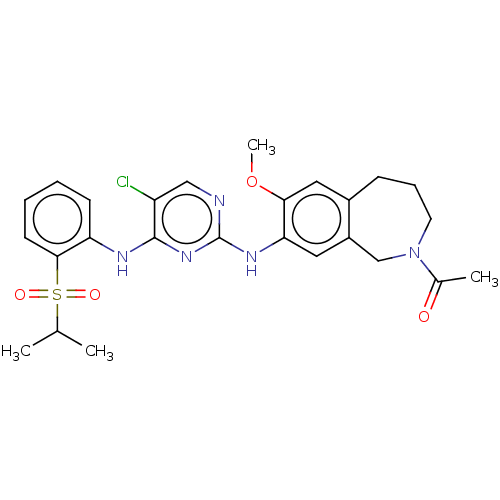 (CHEMBL3608530 | US10053458, 59) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50116686 (CHEMBL3608313 | US10053458, 63) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538131 (US11253516, Example 33) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538132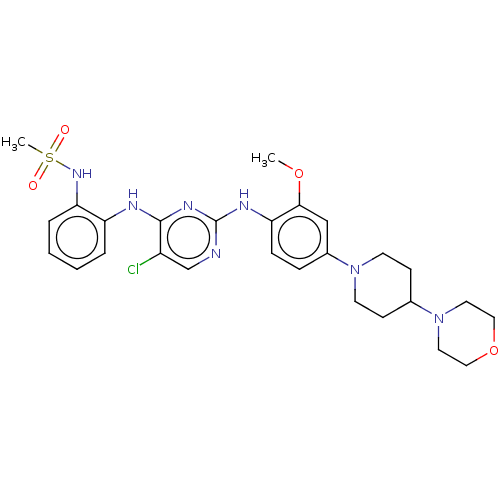 (US11253516, Example 34) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [1-18,20-1210,T790M,C797S] (Homo sapiens (Human)) | BDBM538133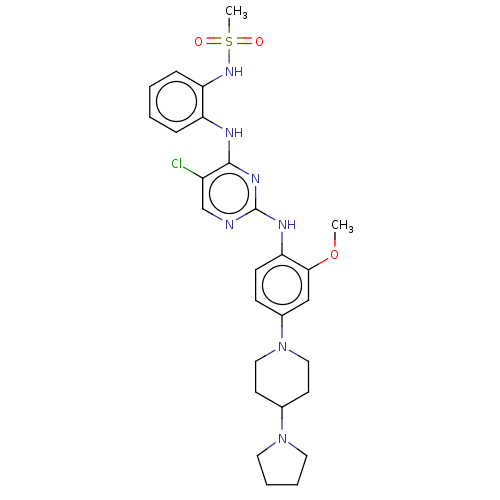 (US11253516, Example 35) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The composition of the assay buffer used in the activity measurement was 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 7.5 mM MgCl2, 3 mM KCl, 0.01% Tween 20, ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RJ4NNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 999 total ) | Next | Last >> |