 Found 26 hits with Last Name = 'hong' and Initial = 'ci'
Found 26 hits with Last Name = 'hong' and Initial = 'ci' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124745
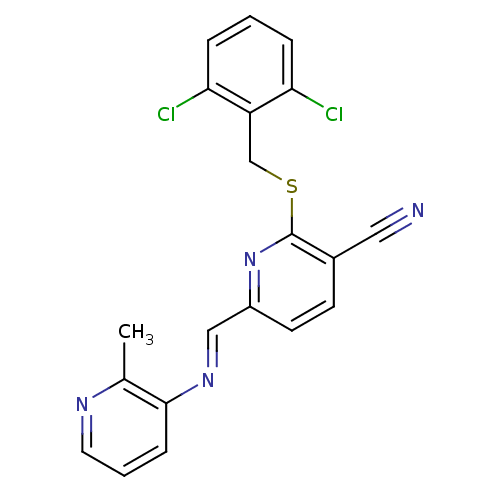
(2-(2,6-Dichloro-benzylsulfanyl)-6-{[(E)-2-methyl-p...)Show SMILES Cc1ncccc1\N=C\c1ccc(C#N)c(SCc2c(Cl)cccc2Cl)n1 Show InChI InChI=1S/C20H14Cl2N4S/c1-13-19(6-3-9-24-13)25-11-15-8-7-14(10-23)20(26-15)27-12-16-17(21)4-2-5-18(16)22/h2-9,11H,12H2,1H3/b25-11+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124726
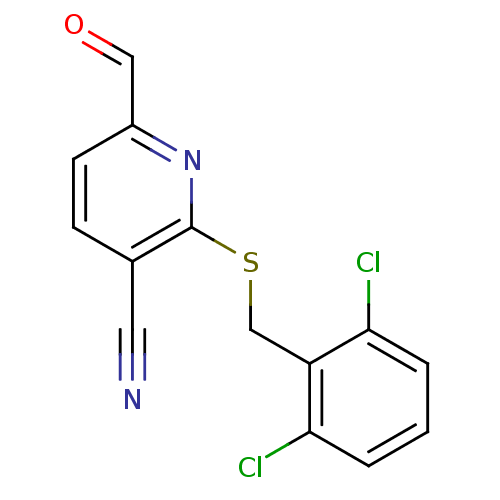
(2-(2,6-Dichloro-benzylsulfanyl)-6-formyl-nicotinon...)Show InChI InChI=1S/C14H8Cl2N2OS/c15-12-2-1-3-13(16)11(12)8-20-14-9(6-17)4-5-10(7-19)18-14/h1-5,7H,8H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro antiproliferative activity of compound against HT-29 (human colon caner ) cell line was determined by SRB assay |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124725
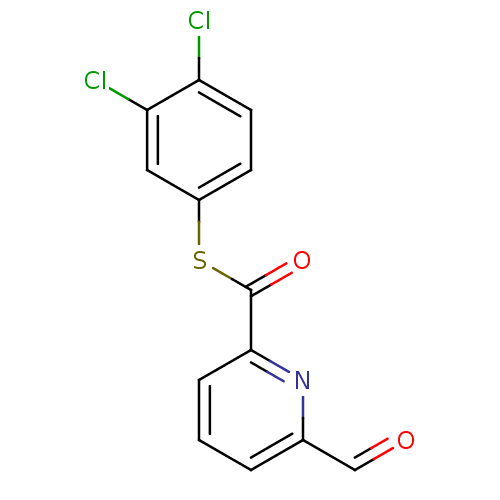
(6-Formyl-pyridine-2-carbothioic acid S-(3,4-dichlo...)Show InChI InChI=1S/C13H7Cl2NO2S/c14-10-5-4-9(6-11(10)15)19-13(18)12-3-1-2-8(7-17)16-12/h1-7H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124734
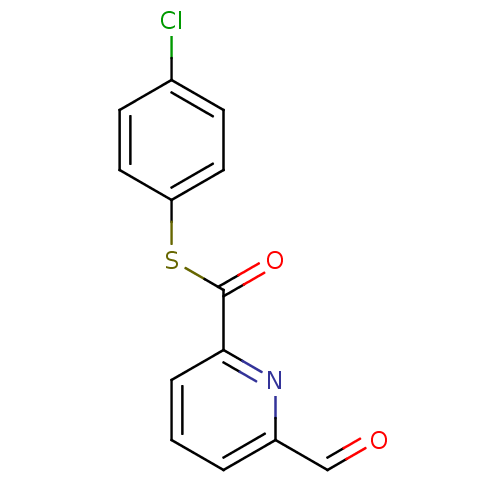
(6-Formyl-pyridine-2-carbothioic acid S-(4-chloro-p...)Show InChI InChI=1S/C13H8ClNO2S/c14-9-4-6-11(7-5-9)18-13(17)12-3-1-2-10(8-16)15-12/h1-8H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124725

(6-Formyl-pyridine-2-carbothioic acid S-(3,4-dichlo...)Show InChI InChI=1S/C13H7Cl2NO2S/c14-10-5-4-9(6-11(10)15)19-13(18)12-3-1-2-8(7-17)16-12/h1-7H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase (Acc Gerons data) |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124737

(6-Formyl-pyridine-2-carbothioic acid 2,4,6-trichlo...)Show InChI InChI=1S/C13H6Cl3NO2S/c14-7-4-9(15)12(10(16)5-7)20-13(19)11-3-1-2-8(6-18)17-11/h1-6H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124742
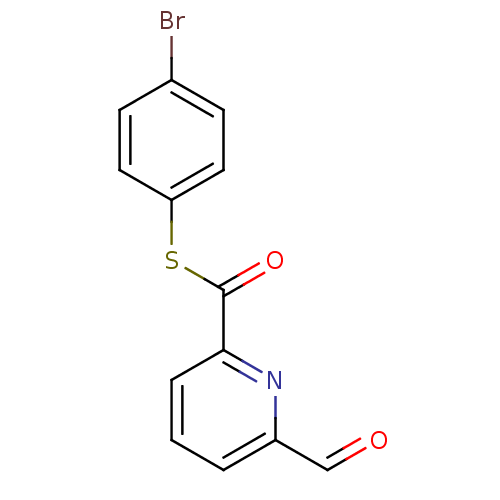
(6-Formyl-pyridine-2-carbothioic acid S-(4-bromo-ph...)Show InChI InChI=1S/C13H8BrNO2S/c14-9-4-6-11(7-5-9)18-13(17)12-3-1-2-10(8-16)15-12/h1-8H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124731
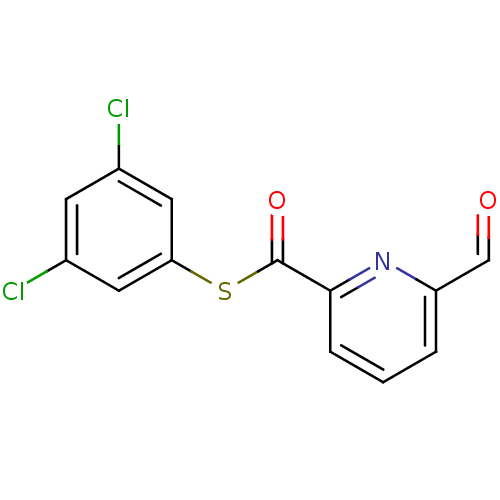
(6-Formyl-pyridine-2-carbothioic acid 3,5-dichloro-...)Show InChI InChI=1S/C13H7Cl2NO2S/c14-8-4-9(15)6-11(5-8)19-13(18)12-3-1-2-10(7-17)16-12/h1-7H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124727

(6-Formyl-pyridine-2-carbothioic acid S-(4-fluoro-p...)Show InChI InChI=1S/C13H8FNO2S/c14-9-4-6-11(7-5-9)18-13(17)12-3-1-2-10(8-16)15-12/h1-8H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124741
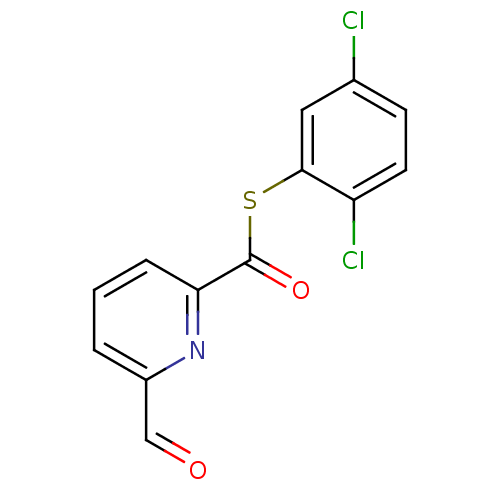
(6-Formyl-pyridine-2-carbothioic acid S-(2,5-dichlo...)Show InChI InChI=1S/C13H7Cl2NO2S/c14-8-4-5-10(15)12(6-8)19-13(18)11-3-1-2-9(7-17)16-11/h1-7H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124736

(6-Formyl-pyridine-2-carbothioic acid S-(3,4-difluo...)Show InChI InChI=1S/C13H7F2NO2S/c14-10-5-4-9(6-11(10)15)19-13(18)12-3-1-2-8(7-17)16-12/h1-7H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124730
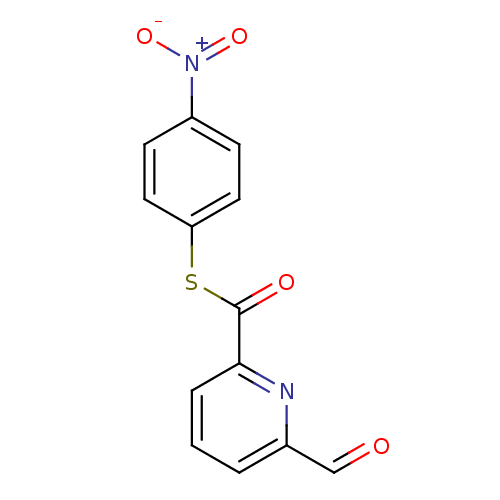
(6-Formyl-pyridine-2-carbothioic acid S-(4-nitro-ph...)Show InChI InChI=1S/C13H8N2O4S/c16-8-9-2-1-3-12(14-9)13(17)20-11-6-4-10(5-7-11)15(18)19/h1-8H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124729

(6-Formyl-pyridine-2-carbothioic acid S-m-tolyl est...)Show InChI InChI=1S/C14H11NO2S/c1-10-4-2-6-12(8-10)18-14(17)13-7-3-5-11(9-16)15-13/h2-9H,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124733
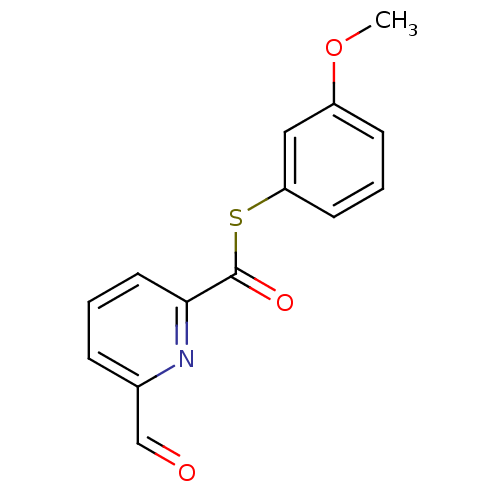
(6-Formyl-pyridine-2-carbothioic acid S-(3-methoxy-...)Show InChI InChI=1S/C14H11NO3S/c1-18-11-5-3-6-12(8-11)19-14(17)13-7-2-4-10(9-16)15-13/h2-9H,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124726

(2-(2,6-Dichloro-benzylsulfanyl)-6-formyl-nicotinon...)Show InChI InChI=1S/C14H8Cl2N2OS/c15-12-2-1-3-13(16)11(12)8-20-14-9(6-17)4-5-10(7-19)18-14/h1-5,7H,8H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124739
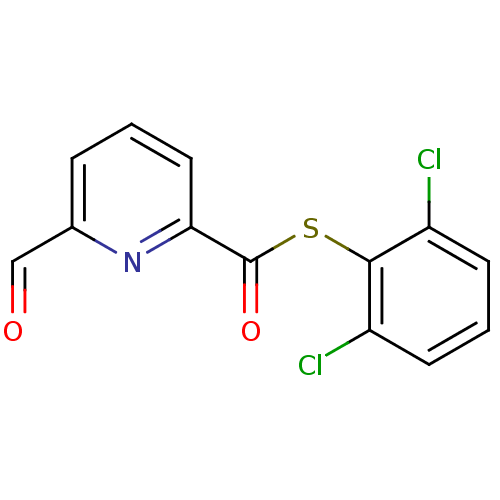
(6-Formyl-pyridine-2-carbothioic acid 2,6-dichloro-...)Show InChI InChI=1S/C13H7Cl2NO2S/c14-9-4-2-5-10(15)12(9)19-13(18)11-6-1-3-8(7-17)16-11/h1-7H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124743

(6-Formyl-pyridine-2-carbothioic acid S-(3-chloro-p...)Show InChI InChI=1S/C13H8ClNO2S/c14-9-3-1-5-11(7-9)18-13(17)12-6-2-4-10(8-16)15-12/h1-8H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124740
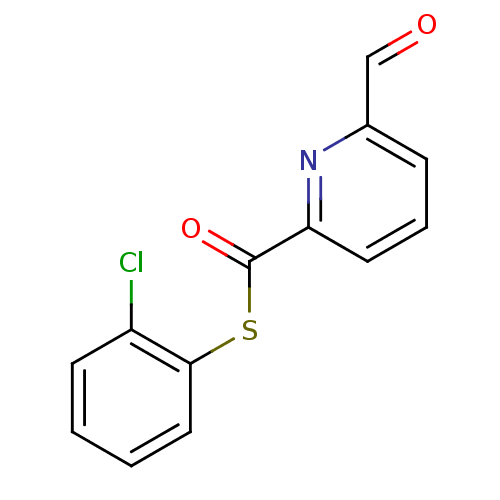
(6-Formyl-pyridine-2-carbothioic acid S-(2-chloro-p...)Show InChI InChI=1S/C13H8ClNO2S/c14-10-5-1-2-7-12(10)18-13(17)11-6-3-4-9(8-16)15-11/h1-8H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124728
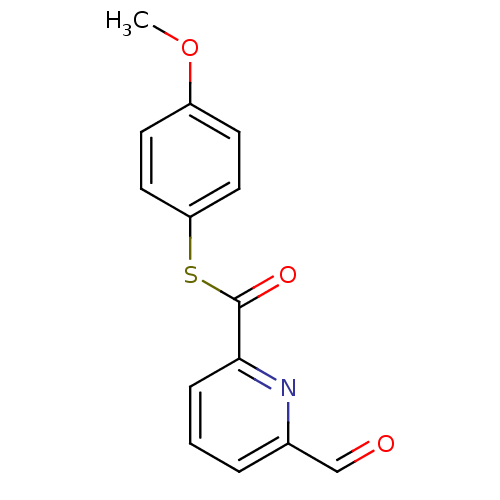
(6-Formyl-pyridine-2-carbothioic acid S-(4-methoxy-...)Show InChI InChI=1S/C14H11NO3S/c1-18-11-5-7-12(8-6-11)19-14(17)13-4-2-3-10(9-16)15-13/h2-9H,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124746
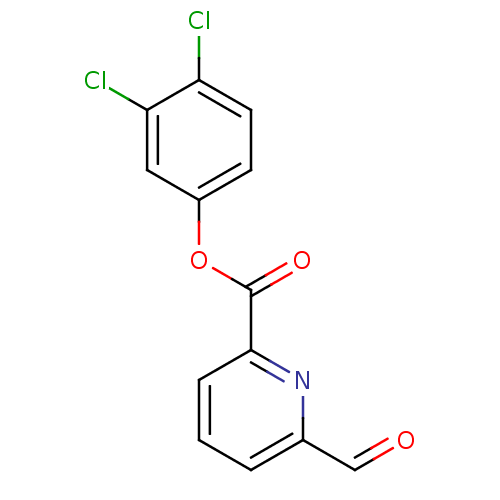
(6-Formyl-pyridine-2-carboxylic acid 3,4-dichloro-p...)Show InChI InChI=1S/C13H7Cl2NO3/c14-10-5-4-9(6-11(10)15)19-13(18)12-3-1-2-8(7-17)16-12/h1-7H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124744

(6-Formyl-pyridine-2-carboxylic acid (3,4-dichloro-...)Show InChI InChI=1S/C13H8Cl2N2O2/c14-10-5-4-8(6-11(10)15)17-13(19)12-3-1-2-9(7-18)16-12/h1-7H,(H,17,19) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124738
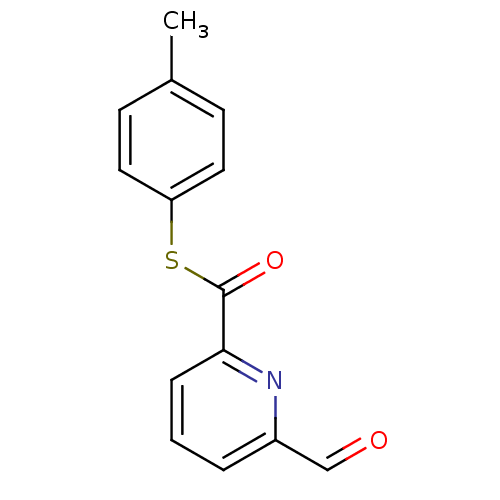
(6-Formyl-pyridine-2-carbothioic acid S-p-tolyl est...)Show InChI InChI=1S/C14H11NO2S/c1-10-5-7-12(8-6-10)18-14(17)13-4-2-3-11(9-16)15-13/h2-9H,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124735
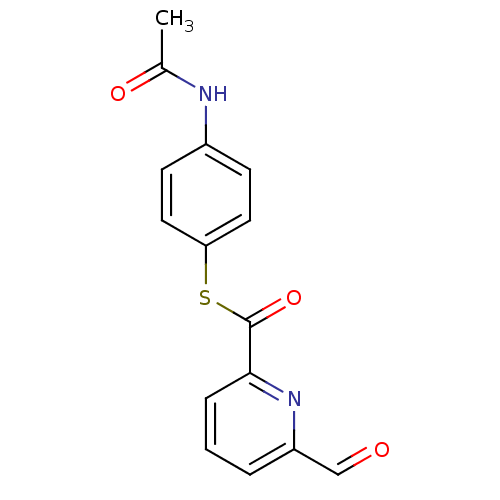
(6-Formyl-pyridine-2-carbothioic acid S-(4-acetylam...)Show InChI InChI=1S/C15H12N2O3S/c1-10(19)16-11-5-7-13(8-6-11)21-15(20)14-4-2-3-12(9-18)17-14/h2-9H,1H3,(H,16,19) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124732
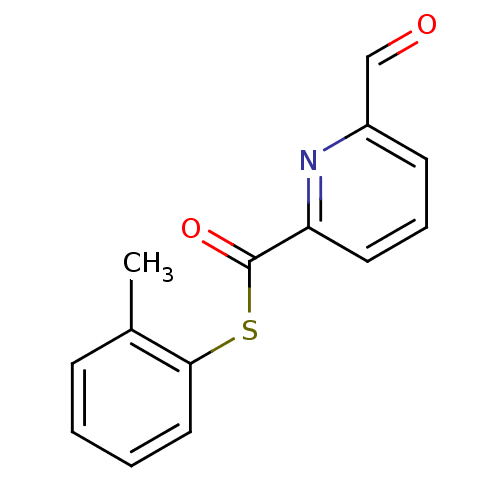
(6-Formyl-pyridine-2-carbothioic acid S-o-tolyl est...)Show InChI InChI=1S/C14H11NO2S/c1-10-5-2-3-8-13(10)18-14(17)12-7-4-6-11(9-16)15-12/h2-9H,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124724
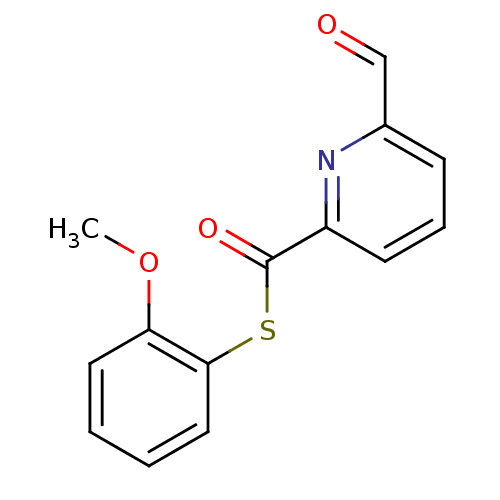
(6-Formyl-pyridine-2-carbothioic acid S-(2-methoxy-...)Show InChI InChI=1S/C14H11NO3S/c1-18-12-7-2-3-8-13(12)19-14(17)11-6-4-5-10(9-16)15-11/h2-9H,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against telomerase |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50124726

(2-(2,6-Dichloro-benzylsulfanyl)-6-formyl-nicotinon...)Show InChI InChI=1S/C14H8Cl2N2OS/c15-12-2-1-3-13(16)11(12)8-20-14-9(6-17)4-5-10(7-19)18-14/h1-5,7H,8H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
In vitro antiproliferative activity of compound against HT-29 (human colon caner ) cell line was determined by SRB assay |
Bioorg Med Chem Lett 13: 609-12 (2003)
BindingDB Entry DOI: 10.7270/Q23R0S7S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data