

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030096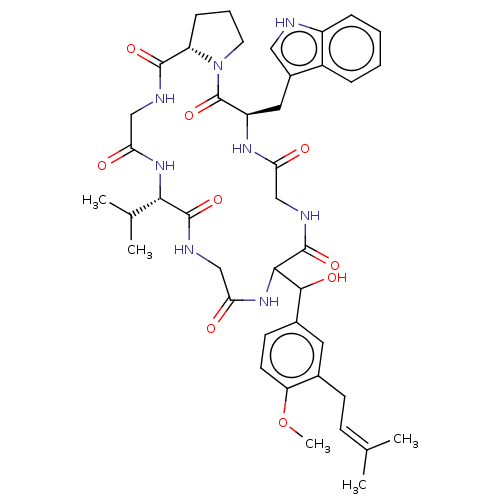 ((R)-11-{Hydroxy-[4-methoxy-3-(3-methyl-but-2-enyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030095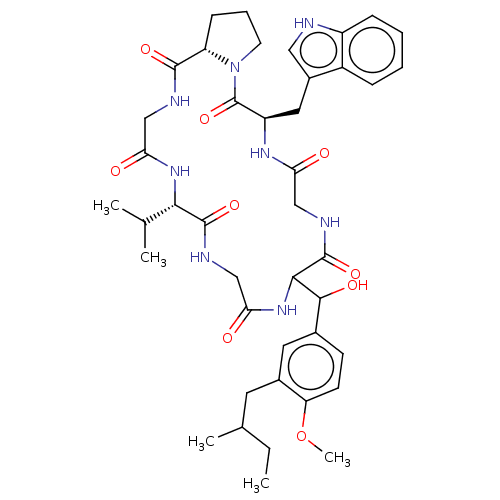 ((R)-11-{Hydroxy-[4-methoxy-3-(2-methyl-butyl)-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030095 ((R)-11-{Hydroxy-[4-methoxy-3-(2-methyl-butyl)-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030096 ((R)-11-{Hydroxy-[4-methoxy-3-(3-methyl-but-2-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030099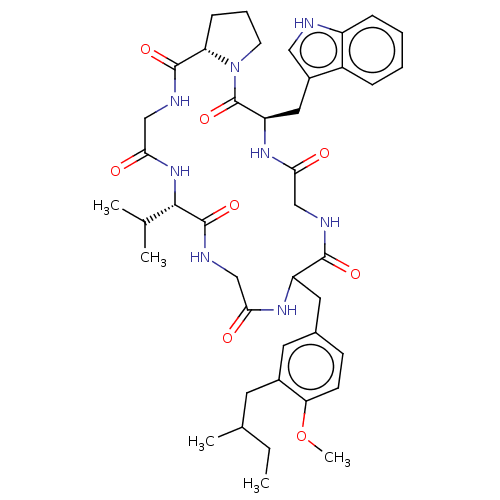 ((R)-5-(1H-Indol-3-ylmethyl)-17-isopropyl-11-[4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030092 ((R)-11-{Hydroxy-[4-hydroxy-3-(3-methyl-but-2-enyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030092 ((R)-11-{Hydroxy-[4-hydroxy-3-(3-methyl-but-2-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030094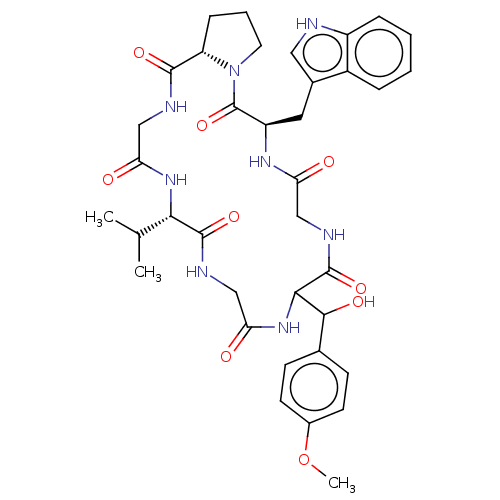 ((R)-11-[Hydroxy-(4-methoxy-phenyl)-methyl]-5-(1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030094 ((R)-11-[Hydroxy-(4-methoxy-phenyl)-methyl]-5-(1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50407271 (CHEMBL2112592) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50407272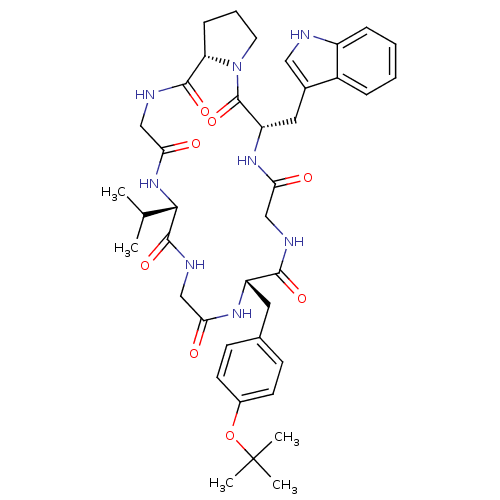 (CHEMBL2111789) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50407271 (CHEMBL2112592) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030091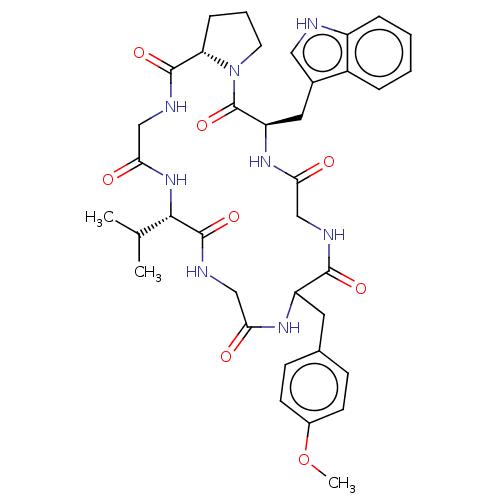 ((R)-5-(1H-Indol-3-ylmethyl)-17-isopropyl-11-(4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030091 ((R)-5-(1H-Indol-3-ylmethyl)-17-isopropyl-11-(4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50407272 (CHEMBL2111789) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50292436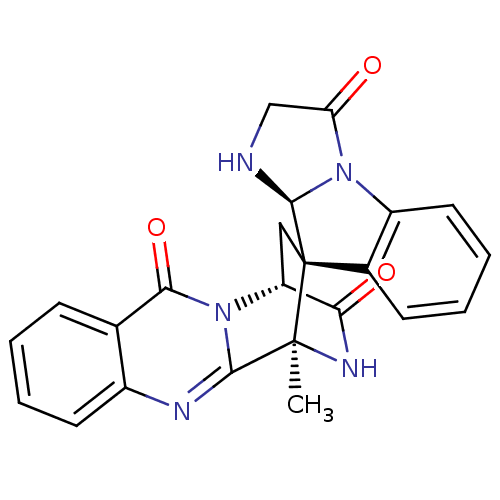 (CHEMBL515179 | spiroquinazoline) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]substance P from NK1 receptor in human astrocytoma cells | J Nat Prod 57: 471-476 (1994) Article DOI: 10.1021/np50106a005 BindingDB Entry DOI: 10.7270/Q2Q2408X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030097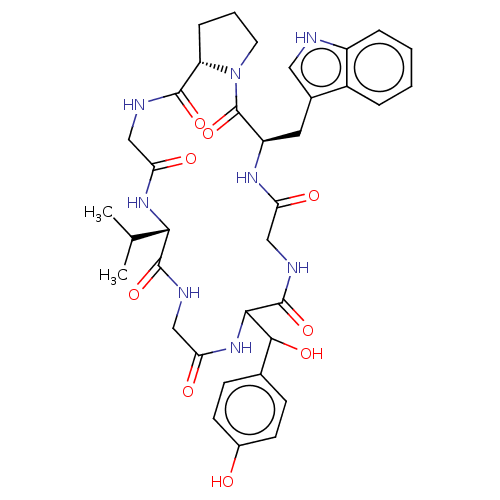 ((R)-11-[Hydroxy-(4-hydroxy-phenyl)-methyl]-5-(1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030098 ((R)-11-(4-Hydroxy-benzyl)-5-(1H-indol-3-ylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030098 ((R)-11-(4-Hydroxy-benzyl)-5-(1H-indol-3-ylmethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030090 ((R)-11-(1H-Indol-5-ylmethyl)-5-(1H-indol-3-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030090 ((R)-11-(1H-Indol-5-ylmethyl)-5-(1H-indol-3-ylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030097 ((R)-11-[Hydroxy-(4-hydroxy-phenyl)-methyl]-5-(1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50292437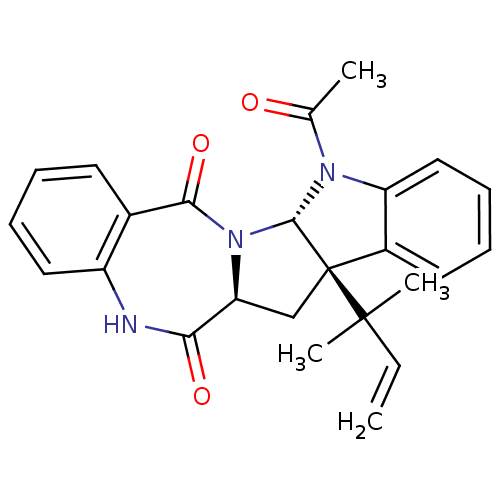 (CHEMBL480101 | acyl aszonalenin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]substance P from NK1 receptor in human astrocytoma cells | J Nat Prod 57: 471-476 (1994) Article DOI: 10.1021/np50106a005 BindingDB Entry DOI: 10.7270/Q2Q2408X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM50412085 (CHEMBL455056) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LFA1 expressed in human JY cells interaction with ICAM1-IG expressed in human HeLa cell monolayer after 45 mins by cell adhesion assay | J Nat Prod 57: 1498-1502 (1994) Article DOI: 10.1021/np50113a004 BindingDB Entry DOI: 10.7270/Q27081GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50292417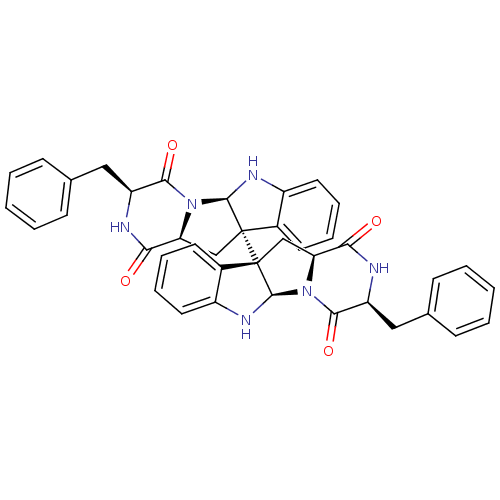 ((3S,5aR,10bR,11aS,3'S,5'R,11'R)-3,3'-Dibenzyl-2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of substance P receptor | J Nat Prod 57: 1239-1244 (1994) Article DOI: 10.1021/np50111a008 BindingDB Entry DOI: 10.7270/Q2GH9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286412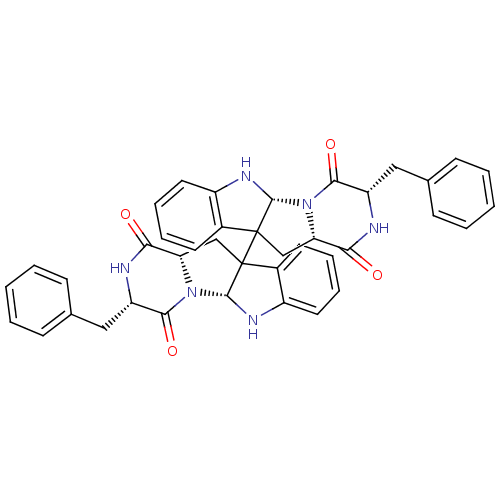 ((3S,5aR,11aS,3'S,5'aR,11'aS)-3,3'-Dibenzyl-2,3,5a,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM50378060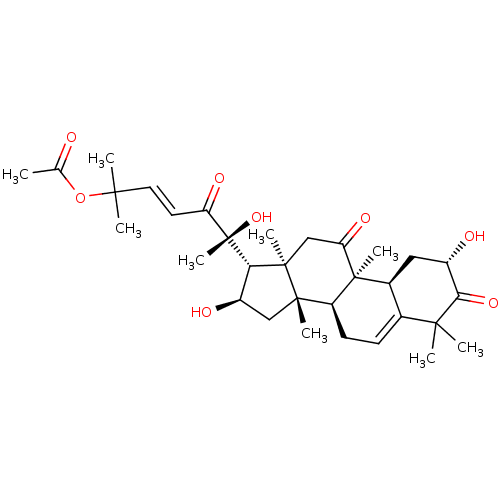 (CUCURBITACIN B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LFA1 expressed in human JY cells interaction with ICAM1-IG expressed in human HeLa cell monolayer after 45 mins by cell adhesion assay | J Nat Prod 57: 1498-1502 (1994) Article DOI: 10.1021/np50113a004 BindingDB Entry DOI: 10.7270/Q27081GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286416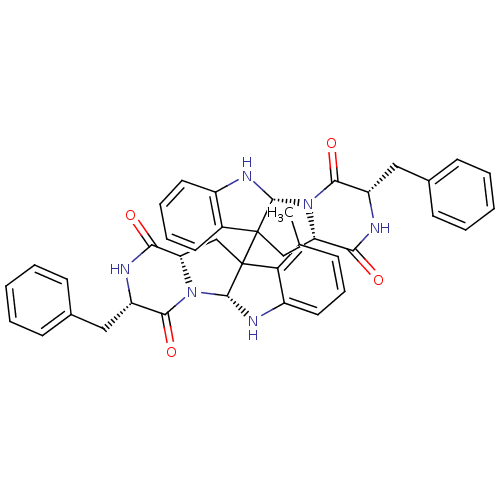 ((3S,5aR,11aS,3'S,5'aR,11'aS)-3,3'-Dibenzyl-10-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM50412087 (CUCURBITACIN I | NSC-521777) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LFA1 expressed in human JY cells interaction with ICAM1-IG expressed in human HeLa cell monolayer after 45 mins by cell adhesion assay | J Nat Prod 57: 1498-1502 (1994) Article DOI: 10.1021/np50113a004 BindingDB Entry DOI: 10.7270/Q27081GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM50412086 (CHEMBL492404) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LFA1 expressed in human JY cells interaction with ICAM1-IG expressed in human HeLa cell monolayer after 45 mins by cell adhesion assay | J Nat Prod 57: 1498-1502 (1994) Article DOI: 10.1021/np50113a004 BindingDB Entry DOI: 10.7270/Q27081GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286409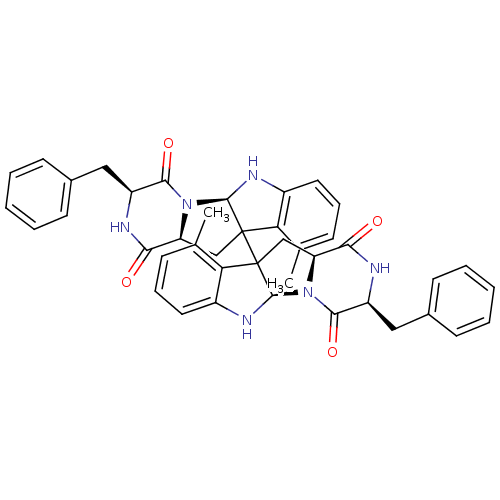 ((3S,5aR,11aS,3'S,5'aR,11'aS)-3,3'-Dibenzyl-10,10'-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50292416 (CHEMBL501675 | Ditryptophenaline) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of substance P receptor | J Nat Prod 57: 1239-1244 (1994) Article DOI: 10.1021/np50111a008 BindingDB Entry DOI: 10.7270/Q2GH9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286418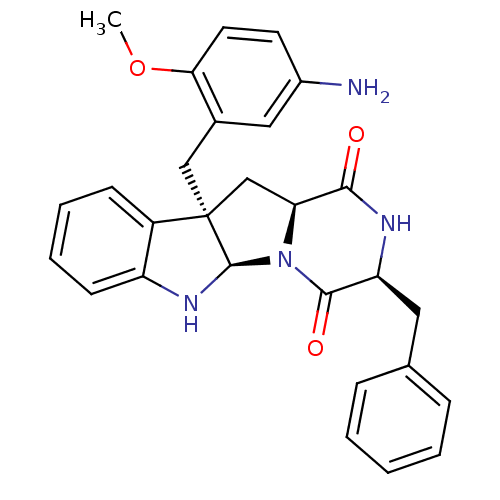 ((3S,5aR,10bR,11aS)-10b-(5-Amino-2-methoxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286410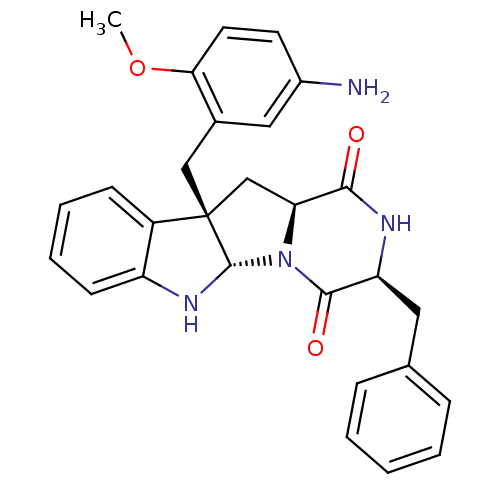 ((3S,5aS,10bS,11aS)-10b-(5-Amino-2-methoxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286411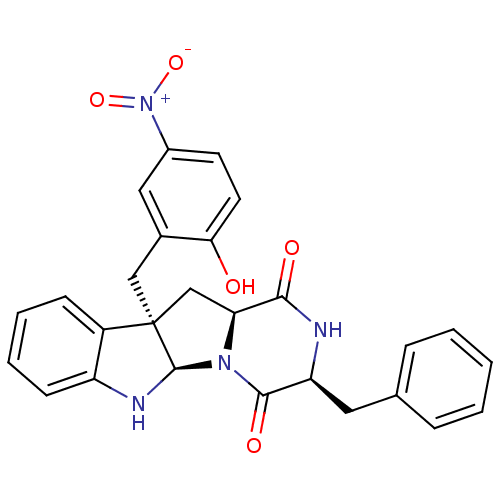 ((3S,5aR,10bR,11aS)-3-Benzyl-10b-(2-hydroxy-5-nitro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM50292420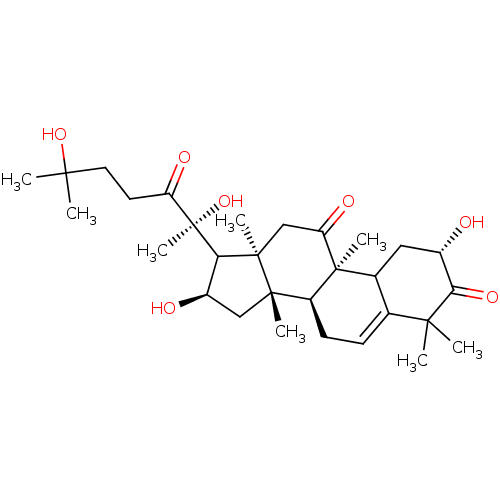 (CHEMBL480112 | Cucurbitacin R) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LFA1 expressed in human JY cells interaction with ICAM1-IG expressed in human HeLa cell monolayer after 45 mins by cell adhesion assay | J Nat Prod 57: 1498-1502 (1994) Article DOI: 10.1021/np50113a004 BindingDB Entry DOI: 10.7270/Q27081GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-L (Homo sapiens (Human)) | BDBM50292421 (CHEMBL480111 | Cucurbitacin L) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LFA1 expressed in human JY cells interaction with ICAM1-IG expressed in human HeLa cell monolayer after 45 mins by cell adhesion assay | J Nat Prod 57: 1498-1502 (1994) Article DOI: 10.1021/np50113a004 BindingDB Entry DOI: 10.7270/Q27081GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286406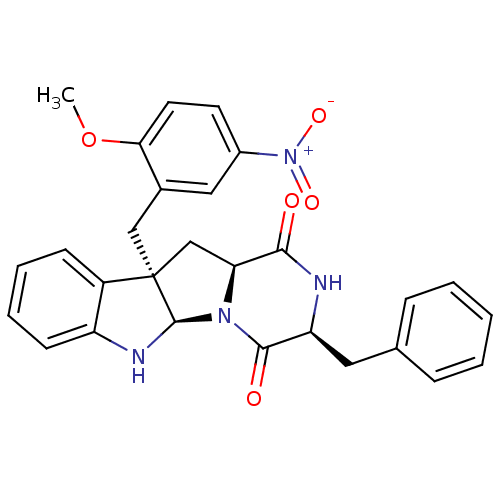 ((3S,5aR,10bR,11aS)-3-Benzyl-10b-(2-methoxy-5-nitro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286417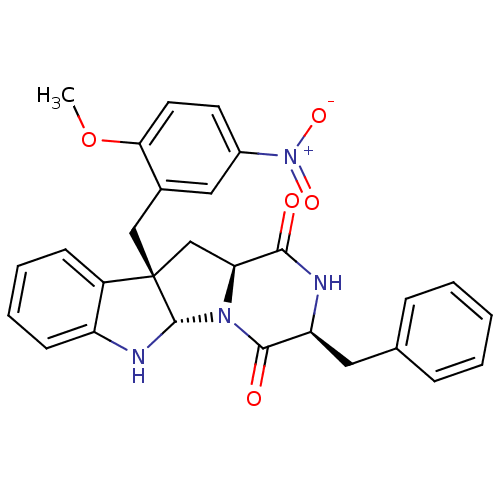 ((3S,5aS,10bS,11aS)-3-Benzyl-10b-(2-methoxy-5-nitro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286413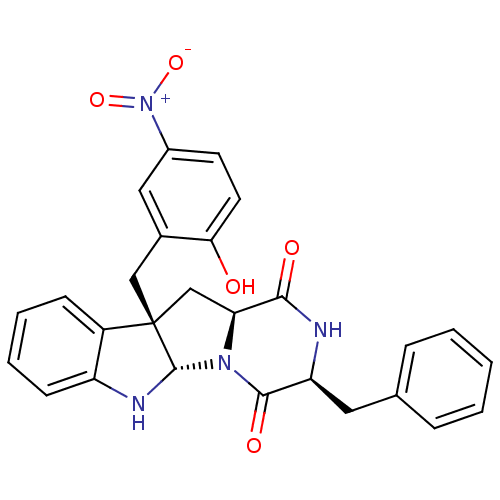 ((3S,5aS,10bS,11aS)-3-Benzyl-10b-(2-hydroxy-5-nitro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286408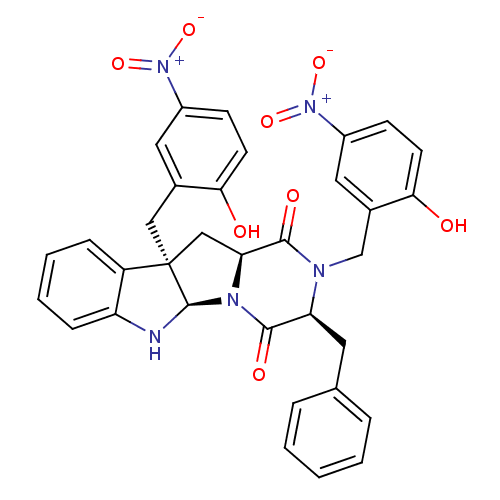 ((3S,5aR,10bR,11aS)-3-Benzyl-2,10b-bis-(2-hydroxy-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286407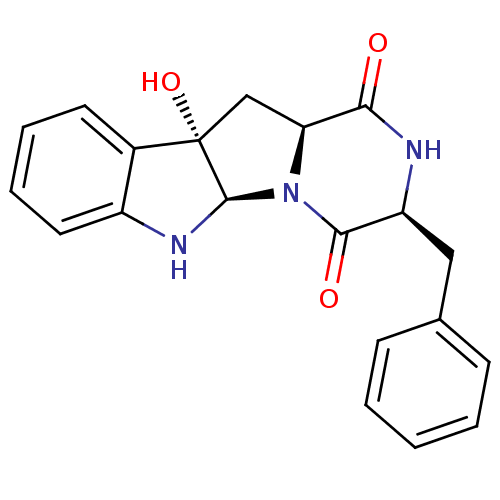 ((3S,5aR,10bS,11aS)-3-Benzyl-10b-hydroxy-2,3,6,10b,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286415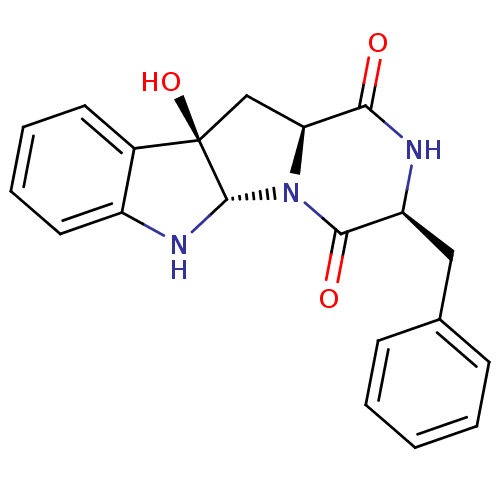 ((3S,5aS,10bR,11aS)-3-Benzyl-10b-hydroxy-2,3,6,10b,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50286414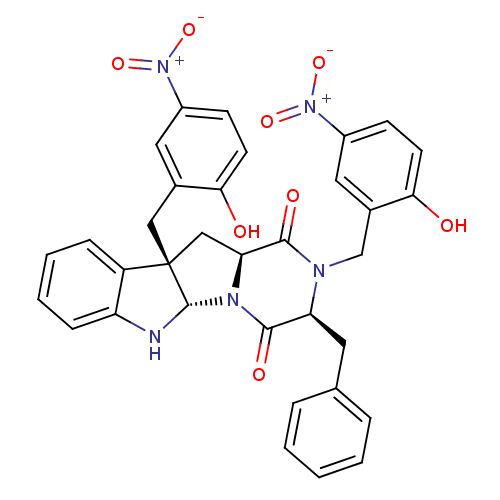 ((3S,5aS,10bS,11aS)-3-Benzyl-2,10b-bis-(2-hydroxy-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonistic activity against Tachykinin receptor 1 | Bioorg Med Chem Lett 5: 377-380 (1995) Article DOI: 10.1016/0960-894X(95)00039-V BindingDB Entry DOI: 10.7270/Q29023R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||