 Found 83 hits with Last Name = 'cowan' and Initial = 'cl'
Found 83 hits with Last Name = 'cowan' and Initial = 'cl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493818

(Oliceridine | TRV-130 | US11124523, Comparative Co...)Show SMILES COc1ccsc1CNCC[C@]1(CCOC2(CCCC2)C1)c1ccccn1 |r| Show InChI InChI=1S/C22H30N2O2S/c1-25-18-7-15-27-19(18)16-23-13-10-21(20-6-2-5-12-24-20)11-14-26-22(17-21)8-3-4-9-22/h2,5-7,12,15,23H,3-4,8-11,13-14,16-17H2,1H3/t21-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human mu opioid receptor by radio-ligand binding assay |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats |
J Med Chem 38: 4670-8 (1995)
Article DOI: 10.1021/jm00023a006
BindingDB Entry DOI: 10.7270/Q21J9DHJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50470654
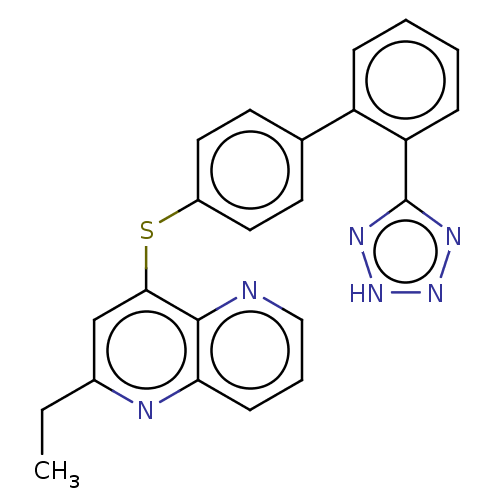
(CHEMBL141019)Show SMILES CCc1cc(Sc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)c2ncccc2n1 Show InChI InChI=1S/C23H18N6S/c1-2-16-14-21(22-20(25-16)8-5-13-24-22)30-17-11-9-15(10-12-17)18-6-3-4-7-19(18)23-26-28-29-27-23/h3-14H,2H2,1H3,(H,26,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats |
J Med Chem 38: 4670-8 (1995)
Article DOI: 10.1021/jm00023a006
BindingDB Entry DOI: 10.7270/Q21J9DHJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50003392
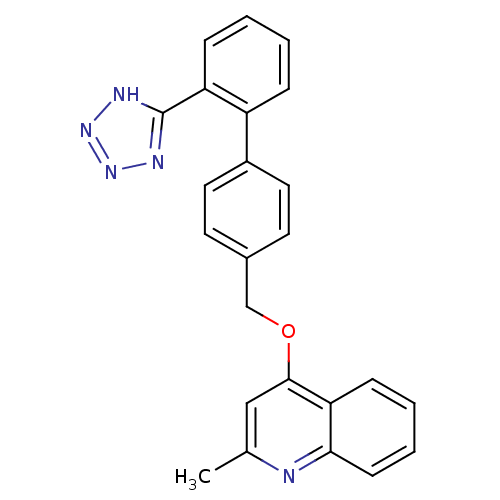
(2-Methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES Cc1cc(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H19N5O/c1-16-14-23(21-8-4-5-9-22(21)25-16)30-15-17-10-12-18(13-11-17)19-6-2-3-7-20(19)24-26-28-29-27-24/h2-14H,15H2,1H3,(H,26,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats |
J Med Chem 38: 4670-8 (1995)
Article DOI: 10.1021/jm00023a006
BindingDB Entry DOI: 10.7270/Q21J9DHJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50029659
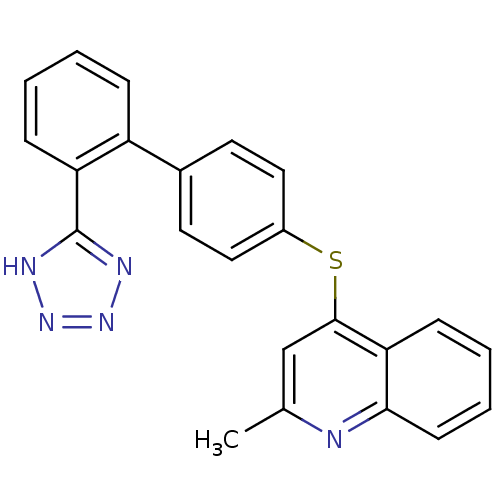
(2-Methyl-4-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylsul...)Show SMILES Cc1cc(Sc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2ccccc2n1 Show InChI InChI=1S/C23H17N5S/c1-15-14-22(20-8-4-5-9-21(20)24-15)29-17-12-10-16(11-13-17)18-6-2-3-7-19(18)23-25-27-28-26-23/h2-14H,1H3,(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats |
J Med Chem 38: 4670-8 (1995)
Article DOI: 10.1021/jm00023a006
BindingDB Entry DOI: 10.7270/Q21J9DHJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50003391
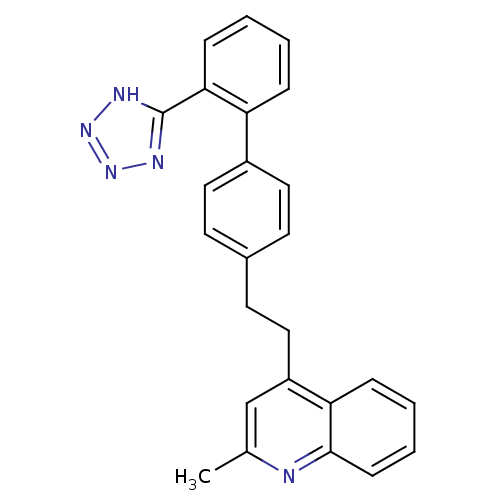
(2-Methyl-4-{2-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...)Show SMILES Cc1cc(CCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H21N5/c1-17-16-20(22-7-4-5-9-24(22)26-17)15-12-18-10-13-19(14-11-18)21-6-2-3-8-23(21)25-27-29-30-28-25/h2-11,13-14,16H,12,15H2,1H3,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats |
J Med Chem 38: 4670-8 (1995)
Article DOI: 10.1021/jm00023a006
BindingDB Entry DOI: 10.7270/Q21J9DHJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50470658
(CHEMBL142409)Show SMILES Cc1nc(Sc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)c2ccccc2n1 Show InChI InChI=1S/C22H16N6S/c1-14-23-20-9-5-4-8-19(20)22(24-14)29-16-12-10-15(11-13-16)17-6-2-3-7-18(17)21-25-27-28-26-21/h2-13H,1H3,(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats |
J Med Chem 38: 4670-8 (1995)
Article DOI: 10.1021/jm00023a006
BindingDB Entry DOI: 10.7270/Q21J9DHJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50003406

(2-Methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES Cc1cc(SCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H19N5S/c1-16-14-23(21-8-4-5-9-22(21)25-16)30-15-17-10-12-18(13-11-17)19-6-2-3-7-20(19)24-26-28-29-27-24/h2-14H,15H2,1H3,(H,26,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats |
J Med Chem 38: 4670-8 (1995)
Article DOI: 10.1021/jm00023a006
BindingDB Entry DOI: 10.7270/Q21J9DHJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50470655
(CHEMBL341724)Show InChI InChI=1S/C19H15N5S/c1-13-12-16(10-11-20-13)25-15-8-6-14(7-9-15)17-4-2-3-5-18(17)19-21-23-24-22-19/h2-12H,1H3,(H,21,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats |
J Med Chem 38: 4670-8 (1995)
Article DOI: 10.1021/jm00023a006
BindingDB Entry DOI: 10.7270/Q21J9DHJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50493811

(CHEMBL2443278)Show SMILES C(C[C@]1(CCOC2(CCCC2)C1)c1ccccn1)NCc1cccs1 |r| Show InChI InChI=1S/C21H28N2OS/c1-4-12-23-19(7-1)20(10-13-22-16-18-6-5-15-25-18)11-14-24-21(17-20)8-2-3-9-21/h1,4-7,12,15,22H,2-3,8-11,13-14,16-17H2/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by whole-cell patch clamp technique |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50493818

(Oliceridine | TRV-130 | US11124523, Comparative Co...)Show SMILES COc1ccsc1CNCC[C@]1(CCOC2(CCCC2)C1)c1ccccn1 |r| Show InChI InChI=1S/C22H30N2O2S/c1-25-18-7-15-27-19(18)16-23-13-10-21(20-6-2-5-12-24-20)11-14-26-22(17-21)8-3-4-9-22/h2,5-7,12,15,23H,3-4,8-11,13-14,16-17H2,1H3/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav 1.5 phasic ion channel expressed in HEK293 cells by whole-cell patch clamp technique |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50470656

(CHEMBL358791)Show SMILES Cc1cc(Sc2ccc(cc2)-c2ccccc2-c2nnn(n2)C(c2ccccc2)(c2ccccc2)c2ccccc2)c2ccccc2n1 Show InChI InChI=1S/C42H31N5S/c1-30-29-40(38-23-13-14-24-39(38)43-30)48-35-27-25-31(26-28-35)36-21-11-12-22-37(36)41-44-46-47(45-41)42(32-15-5-2-6-16-32,33-17-7-3-8-18-33)34-19-9-4-10-20-34/h2-29H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats |
J Med Chem 38: 4670-8 (1995)
Article DOI: 10.1021/jm00023a006
BindingDB Entry DOI: 10.7270/Q21J9DHJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50493818

(Oliceridine | TRV-130 | US11124523, Comparative Co...)Show SMILES COc1ccsc1CNCC[C@]1(CCOC2(CCCC2)C1)c1ccccn1 |r| Show InChI InChI=1S/C22H30N2O2S/c1-25-18-7-15-27-19(18)16-23-13-10-21(20-6-2-5-12-24-20)11-14-26-22(17-21)8-3-4-9-22/h2,5-7,12,15,23H,3-4,8-11,13-14,16-17H2,1H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by whole-cell patch clamp technique |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50493811

(CHEMBL2443278)Show SMILES C(C[C@]1(CCOC2(CCCC2)C1)c1ccccn1)NCc1cccs1 |r| Show InChI InChI=1S/C21H28N2OS/c1-4-12-23-19(7-1)20(10-13-22-16-18-6-5-15-25-18)11-14-24-21(17-20)8-2-3-9-21/h1,4-7,12,15,22H,2-3,8-11,13-14,16-17H2/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav 1.5 phasic ion channel expressed in HEK293 cells by whole-cell patch clamp technique |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50470659
(CHEMBL358044)Show SMILES Cc1cc(c2ccccc2[n+]1[O-])S(=O)(=O)c1ccc(cc1)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C23H17N5O3S/c1-15-14-22(20-8-4-5-9-21(20)28(15)29)32(30,31)17-12-10-16(11-13-17)18-6-2-3-7-19(18)23-24-26-27-25-23/h2-14H,1H3,(H,24,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats |
J Med Chem 38: 4670-8 (1995)
Article DOI: 10.1021/jm00023a006
BindingDB Entry DOI: 10.7270/Q21J9DHJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50470657
(CHEMBL142300)Show SMILES Cc1nc(Sc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)c2cc[nH]c2n1 Show InChI InChI=1S/C20H15N7S/c1-12-22-18-17(10-11-21-18)20(23-12)28-14-8-6-13(7-9-14)15-4-2-3-5-16(15)19-24-26-27-25-19/h2-11H,1H3,(H,21,22,23)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AII from the Angiotensin II receptor isolated from the liver of rats |
J Med Chem 38: 4670-8 (1995)
Article DOI: 10.1021/jm00023a006
BindingDB Entry DOI: 10.7270/Q21J9DHJ |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50493818

(Oliceridine | TRV-130 | US11124523, Comparative Co...)Show SMILES COc1ccsc1CNCC[C@]1(CCOC2(CCCC2)C1)c1ccccn1 |r| Show InChI InChI=1S/C22H30N2O2S/c1-25-18-7-15-27-19(18)16-23-13-10-21(20-6-2-5-12-24-20)11-14-26-22(17-21)8-3-4-9-22/h2,5-7,12,15,23H,3-4,8-11,13-14,16-17H2,1H3/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav 1.5 tonic ion channel expressed in HEK293 cells by whole-cell patch clamp technique |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50493811

(CHEMBL2443278)Show SMILES C(C[C@]1(CCOC2(CCCC2)C1)c1ccccn1)NCc1cccs1 |r| Show InChI InChI=1S/C21H28N2OS/c1-4-12-23-19(7-1)20(10-13-22-16-18-6-5-15-25-18)11-14-24-21(17-20)8-2-3-9-21/h1,4-7,12,15,22H,2-3,8-11,13-14,16-17H2/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Nav 1.5 tonic ion channel expressed in HEK293 cells by whole-cell patch clamp technique |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50493818

(Oliceridine | TRV-130 | US11124523, Comparative Co...)Show SMILES COc1ccsc1CNCC[C@]1(CCOC2(CCCC2)C1)c1ccccn1 |r| Show InChI InChI=1S/C22H30N2O2S/c1-25-18-7-15-27-19(18)16-23-13-10-21(20-6-2-5-12-24-20)11-14-26-22(17-21)8-3-4-9-22/h2,5-7,12,15,23H,3-4,8-11,13-14,16-17H2,1H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Cav 1.2 ion channel expressed in HEK293 cells by whole-cell patch clamp technique |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50493811

(CHEMBL2443278)Show SMILES C(C[C@]1(CCOC2(CCCC2)C1)c1ccccn1)NCc1cccs1 |r| Show InChI InChI=1S/C21H28N2OS/c1-4-12-23-19(7-1)20(10-13-22-16-18-6-5-15-25-18)11-14-24-21(17-20)8-2-3-9-21/h1,4-7,12,15,22H,2-3,8-11,13-14,16-17H2/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Cav 1.2 ion channel expressed in HEK293 cells by whole-cell patch clamp technique |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493801

(CHEMBL2443256)Show SMILES Cc1csc(CNCC[C@]2(CCOC3(CCCC3)C2)c2ccccn2)c1 |r| Show InChI InChI=1S/C22H30N2OS/c1-18-14-19(26-16-18)15-23-12-9-21(20-6-2-5-11-24-20)10-13-25-22(17-21)7-3-4-8-22/h2,5-6,11,14,16,23H,3-4,7-10,12-13,15,17H2,1H3/t21-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 398 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as beta-arrestin recruitment by chemiluminescence assay |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493802

(CHEMBL2443272)Show SMILES Clc1cccc(CNCC[C@]2(CCOC3(CCCC3)C2)c2ccccn2)c1 |r| Show InChI InChI=1S/C23H29ClN2O/c24-20-7-5-6-19(16-20)17-25-14-11-22(21-8-1-4-13-26-21)12-15-27-23(18-22)9-2-3-10-23/h1,4-8,13,16,25H,2-3,9-12,14-15,17-18H2/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as beta-arrestin recruitment by chemiluminescence assay |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493803

(CHEMBL2443280)Show SMILES CC[C@]1(C)CC(CCNCc2ccccc2O)(CCO1)c1ccc(C)cc1 |r| Show InChI InChI=1S/C24H33NO2/c1-4-23(3)18-24(14-16-27-23,21-11-9-19(2)10-12-21)13-15-25-17-20-7-5-6-8-22(20)26/h5-12,25-26H,4,13-18H2,1-3H3/t23-,24?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as beta-arrestin recruitment by chemiluminescence assay |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493804

(CHEMBL2443264)Show SMILES Fc1ccc(cc1)[C@@]1(CCNCc2ccccc2)CCOC2(CCCC2)C1 |r| Show InChI InChI=1S/C24H30FNO/c25-22-10-8-21(9-11-22)23(14-16-26-18-20-6-2-1-3-7-20)15-17-27-24(19-23)12-4-5-13-24/h1-3,6-11,26H,4-5,12-19H2/t23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as beta-arrestin recruitment by chemiluminescence assay |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493803

(CHEMBL2443280)Show SMILES CC[C@]1(C)CC(CCNCc2ccccc2O)(CCO1)c1ccc(C)cc1 |r| Show InChI InChI=1S/C24H33NO2/c1-4-23(3)18-24(14-16-27-23,21-11-9-19(2)10-12-21)13-15-25-17-20-7-5-6-8-22(20)26/h5-12,25-26H,4,13-18H2,1-3H3/t23-,24?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493805
(CHEMBL2443271)Show SMILES Clc1ccccc1CNCC[C@]1(CCOC2(CCCC2)C1)c1ccccn1 |r| Show InChI InChI=1S/C23H29ClN2O/c24-20-8-2-1-7-19(20)17-25-15-12-22(21-9-3-6-14-26-21)13-16-27-23(18-22)10-4-5-11-23/h1-3,6-9,14,25H,4-5,10-13,15-18H2/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493806
(CHEMBL2443254)Show SMILES C(C[C@]1(CCOC2(CCCC2)C1)c1ccccn1)NCC1CCCC1 |r| Show InChI InChI=1S/C22H34N2O/c1-2-8-19(7-1)17-23-15-12-21(20-9-3-6-14-24-20)13-16-25-22(18-21)10-4-5-11-22/h3,6,9,14,19,23H,1-2,4-5,7-8,10-13,15-18H2/t21-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493807

(CHEMBL2443261)Show SMILES Cc1cc(CNCC[C@]2(CCOC3(CCCC3)C2)c2ccccn2)oc1C |r| Show InChI InChI=1S/C23H32N2O2/c1-18-15-20(27-19(18)2)16-24-13-10-22(21-7-3-6-12-25-21)11-14-26-23(17-22)8-4-5-9-23/h3,6-7,12,15,24H,4-5,8-11,13-14,16-17H2,1-2H3/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493808
(CHEMBL2443257)Show SMILES Cc1ccc(CNCC[C@]2(CCOC3(CCCC3)C2)c2ccccn2)s1 |r| Show InChI InChI=1S/C22H30N2OS/c1-18-7-8-19(26-18)16-23-14-11-21(20-6-2-5-13-24-20)12-15-25-22(17-21)9-3-4-10-22/h2,5-8,13,23H,3-4,9-12,14-17H2,1H3/t21-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493801

(CHEMBL2443256)Show SMILES Cc1csc(CNCC[C@]2(CCOC3(CCCC3)C2)c2ccccn2)c1 |r| Show InChI InChI=1S/C22H30N2OS/c1-18-14-19(26-16-18)15-23-12-9-21(20-6-2-5-11-24-20)10-13-25-22(17-21)7-3-4-8-22/h2,5-6,11,14,16,23H,3-4,7-10,12-13,15,17H2,1H3/t21-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493809

(CHEMBL2443253)Show SMILES C(C[C@]1(CCOC2(CCCC2)C1)c1ccccn1)NCc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C27H32N2O/c1-2-8-24-19-22(10-11-23(24)7-1)20-28-17-14-26(25-9-3-6-16-29-25)15-18-30-27(21-26)12-4-5-13-27/h1-3,6-11,16,19,28H,4-5,12-15,17-18,20-21H2/t26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493810

(CHEMBL2443279)Show SMILES C(C[C@]1(CCOC2(CCCC2)C1)c1ccccn1)NCc1ccsc1 |r| Show InChI InChI=1S/C21H28N2OS/c1-4-11-23-19(5-1)20(9-12-22-15-18-6-14-25-16-18)10-13-24-21(17-20)7-2-3-8-21/h1,4-6,11,14,16,22H,2-3,7-10,12-13,15,17H2/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493811

(CHEMBL2443278)Show SMILES C(C[C@]1(CCOC2(CCCC2)C1)c1ccccn1)NCc1cccs1 |r| Show InChI InChI=1S/C21H28N2OS/c1-4-12-23-19(7-1)20(10-13-22-16-18-6-5-15-25-18)11-14-24-21(17-20)8-2-3-9-21/h1,4-7,12,15,22H,2-3,8-11,13-14,16-17H2/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493802

(CHEMBL2443272)Show SMILES Clc1cccc(CNCC[C@]2(CCOC3(CCCC3)C2)c2ccccn2)c1 |r| Show InChI InChI=1S/C23H29ClN2O/c24-20-7-5-6-19(16-20)17-25-14-11-22(21-8-1-4-13-26-21)12-15-27-23(18-22)9-2-3-10-23/h1,4-8,13,16,25H,2-3,9-12,14-15,17-18H2/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493812

(CHEMBL2443268)Show InChI InChI=1S/C23H30N2O/c1-2-8-20(9-3-1)18-24-16-13-22(21-10-4-7-15-25-21)14-17-26-23(19-22)11-5-6-12-23/h1-4,7-10,15,24H,5-6,11-14,16-19H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493813
(CHEMBL2440150)Show SMILES CC(C)(NCC[C@]1(CCOC2(CCCC2)C1)c1ccc(F)cc1)c1ccccc1 |r| Show InChI InChI=1S/C26H34FNO/c1-24(2,21-8-4-3-5-9-21)28-18-16-25(22-10-12-23(27)13-11-22)17-19-29-26(20-25)14-6-7-15-26/h3-5,8-13,28H,6-7,14-20H2,1-2H3/t25-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493814

(CHEMBL2443266)Show SMILES C[C@H](NCC[C@]1(CCOC2(CCCC2)C1)c1ccc(F)cc1)c1ccccc1 |r| Show InChI InChI=1S/C25H32FNO/c1-20(21-7-3-2-4-8-21)27-17-15-24(22-9-11-23(26)12-10-22)16-18-28-25(19-24)13-5-6-14-25/h2-4,7-12,20,27H,5-6,13-19H2,1H3/t20-,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493804

(CHEMBL2443264)Show SMILES Fc1ccc(cc1)[C@@]1(CCNCc2ccccc2)CCOC2(CCCC2)C1 |r| Show InChI InChI=1S/C24H30FNO/c25-22-10-8-21(9-11-22)23(14-16-26-18-20-6-2-1-3-7-20)15-17-27-24(19-23)12-4-5-13-24/h1-3,6-11,26H,4-5,12-19H2/t23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493815
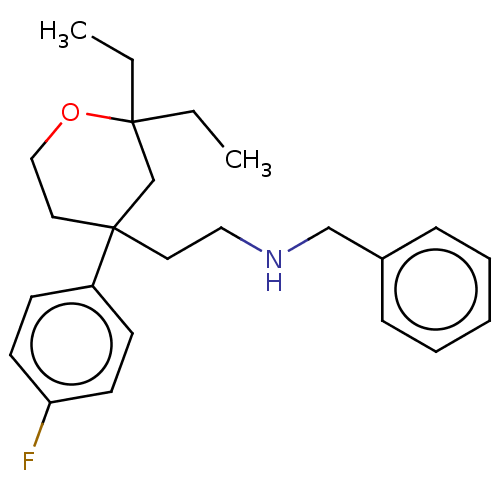
(CHEMBL2443284)Show InChI InChI=1S/C24H32FNO/c1-3-24(4-2)19-23(15-17-27-24,21-10-12-22(25)13-11-21)14-16-26-18-20-8-6-5-7-9-20/h5-13,26H,3-4,14-19H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493816

(CHEMBL2443283)Show InChI InChI=1S/C22H28FNO/c1-21(2)17-22(13-15-25-21,19-8-10-20(23)11-9-19)12-14-24-16-18-6-4-3-5-7-18/h3-11,24H,12-17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493817

(CHEMBL2443281)Show InChI InChI=1S/C21H27NO2/c1-17-6-8-19(9-7-17)21(11-14-24-15-12-21)10-13-22-16-18-4-2-3-5-20(18)23/h2-9,22-23H,10-16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluorescen... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50493818

(Oliceridine | TRV-130 | US11124523, Comparative Co...)Show SMILES COc1ccsc1CNCC[C@]1(CCOC2(CCCC2)C1)c1ccccn1 |r| Show InChI InChI=1S/C22H30N2O2S/c1-25-18-7-15-27-19(18)16-23-13-10-21(20-6-2-5-12-24-20)11-14-26-22(17-21)8-3-4-9-22/h2,5-7,12,15,23H,3-4,8-11,13-14,16-17H2,1H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluores... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50493811

(CHEMBL2443278)Show SMILES C(C[C@]1(CCOC2(CCCC2)C1)c1ccccn1)NCc1cccs1 |r| Show InChI InChI=1S/C21H28N2OS/c1-4-12-23-19(7-1)20(10-13-22-16-18-6-5-15-25-18)11-14-24-21(17-20)8-2-3-9-21/h1,4-7,12,15,22H,2-3,8-11,13-14,16-17H2/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation by fluores... |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493807

(CHEMBL2443261)Show SMILES Cc1cc(CNCC[C@]2(CCOC3(CCCC3)C2)c2ccccn2)oc1C |r| Show InChI InChI=1S/C23H32N2O2/c1-18-15-20(27-19(18)2)16-24-13-10-22(21-7-3-6-12-25-21)11-14-26-23(17-22)8-4-5-9-23/h3,6-7,12,15,24H,4-5,8-11,13-14,16-17H2,1-2H3/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as beta-arrestin recruitment by chemiluminescence assay |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493819

(CHEMBL2443259)Show SMILES Cc1cc(C)c(CNCC[C@]2(CCOC3(CCCC3)C2)c2ccccn2)s1 |r| Show InChI InChI=1S/C23H32N2OS/c1-18-15-19(2)27-20(18)16-24-13-10-22(21-7-3-6-12-25-21)11-14-26-23(17-22)8-4-5-9-23/h3,6-7,12,15,24H,4-5,8-11,13-14,16-17H2,1-2H3/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 631 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as beta-arrestin recruitment by chemiluminescence assay |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493809

(CHEMBL2443253)Show SMILES C(C[C@]1(CCOC2(CCCC2)C1)c1ccccn1)NCc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C27H32N2O/c1-2-8-24-19-22(10-11-23(24)7-1)20-28-17-14-26(25-9-3-6-16-29-25)15-18-30-27(21-26)12-4-5-13-27/h1-3,6-11,16,19,28H,4-5,12-15,17-18,20-21H2/t26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as beta-arrestin recruitment by chemiluminescence assay |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493810

(CHEMBL2443279)Show SMILES C(C[C@]1(CCOC2(CCCC2)C1)c1ccccn1)NCc1ccsc1 |r| Show InChI InChI=1S/C21H28N2OS/c1-4-11-23-19(5-1)20(9-12-22-15-18-6-14-25-16-18)10-13-24-21(17-20)7-2-3-8-21/h1,4-6,11,14,16,22H,2-3,7-10,12-13,15,17H2/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as beta-arrestin recruitment by chemiluminescence assay |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493820

(CHEMBL2443277)Show SMILES C(C[C@]1(CCOC2(CCCC2)C1)c1ccccn1)NCc1ccncn1 |r| Show InChI InChI=1S/C21H28N4O/c1-4-11-24-19(5-1)20(9-13-22-15-18-6-12-23-17-25-18)10-14-26-21(16-20)7-2-3-8-21/h1,4-6,11-12,17,22H,2-3,7-10,13-16H2/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as beta-arrestin recruitment by chemiluminescence assay |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50493812

(CHEMBL2443268)Show InChI InChI=1S/C23H30N2O/c1-2-8-20(9-3-1)18-24-16-13-22(21-10-4-7-15-25-21)14-17-26-23(19-22)11-5-6-12-23/h1-4,7-10,15,24H,5-6,11-14,16-19H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Trevena, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as beta-arrestin recruitment by chemiluminescence assay |
J Med Chem 56: 8019-31 (2013)
Article DOI: 10.1021/jm4010829
BindingDB Entry DOI: 10.7270/Q2FT8Q0N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data