

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430681 (CHEMBL2332979) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430652 (CHEMBL2332975) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430680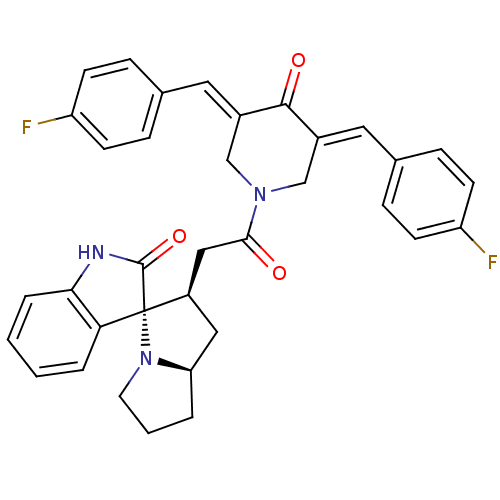 (CHEMBL2332980) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430683 (CHEMBL2332977) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430651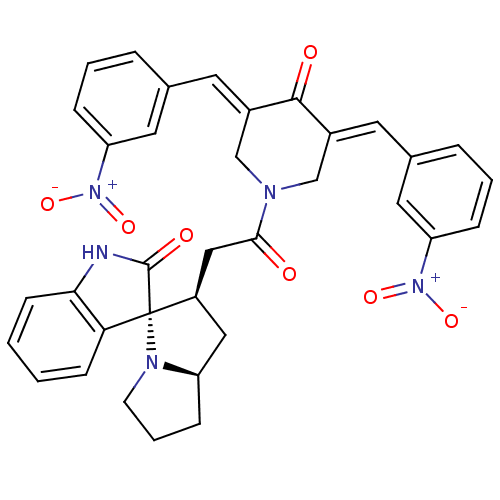 (CHEMBL2332976) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430668 (CHEMBL2332981) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430679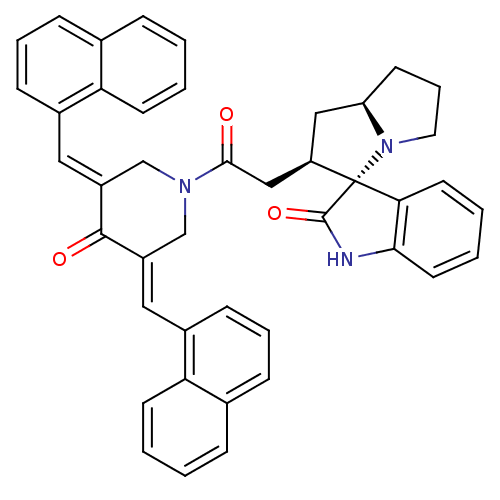 (CHEMBL2332545) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430672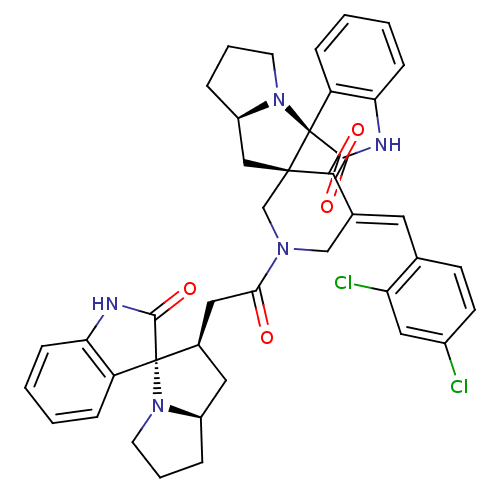 (CHEMBL2332531) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430670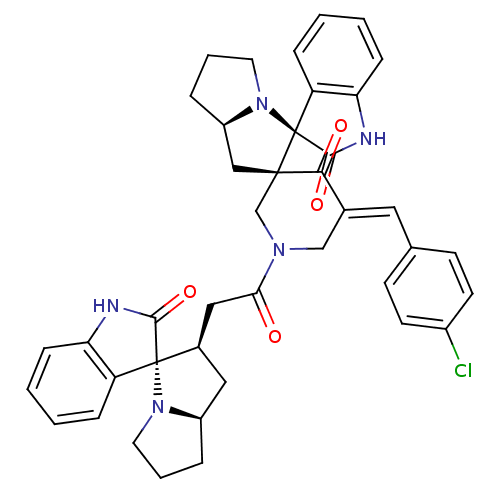 (CHEMBL2332541) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430669 (CHEMBL2332540) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430665 (CHEMBL2332539) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430674 (CHEMBL2332533) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430654 (CHEMBL2332550) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430672 (CHEMBL2332531) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430662 (CHEMBL2332537) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430670 (CHEMBL2332541) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430676 (CHEMBL2332542) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430675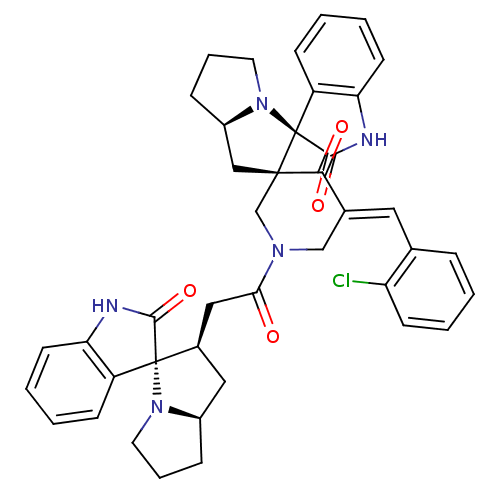 (CHEMBL2332534) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430679 (CHEMBL2332545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430663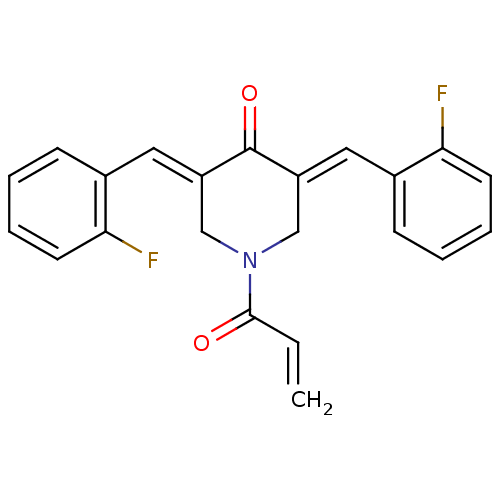 (CHEMBL2332536) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430653 (CHEMBL2332974) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430655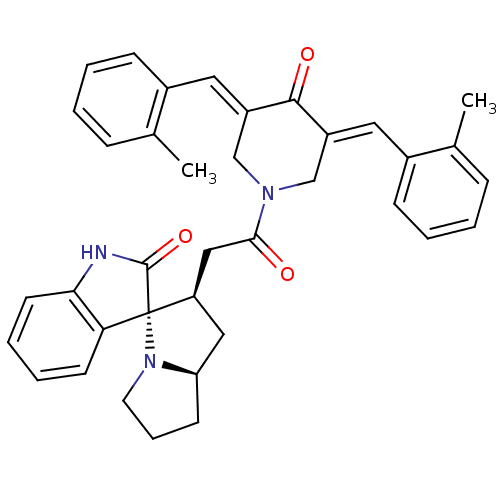 (CHEMBL2332549) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430677 (CHEMBL2332543) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430675 (CHEMBL2332534) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430657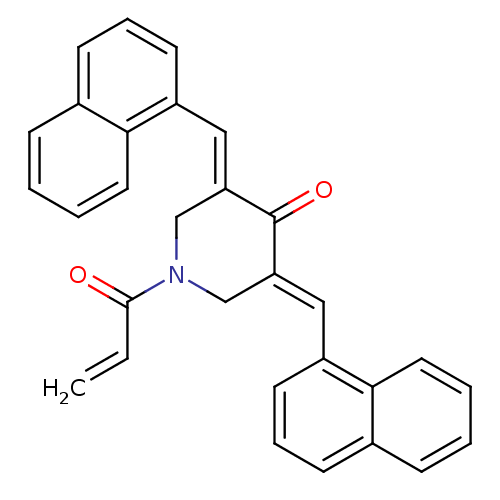 (CHEMBL2332547) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430658 (CHEMBL350032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430661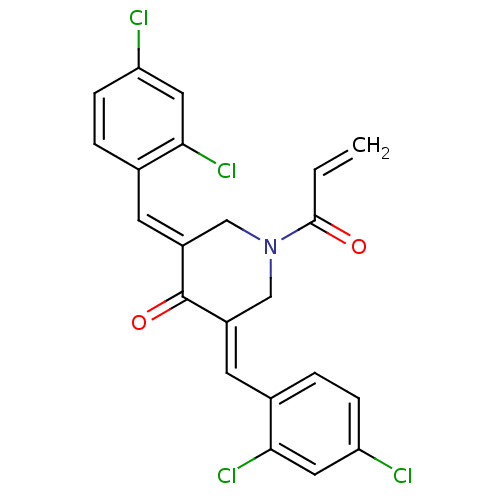 (CHEMBL2332546) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430654 (CHEMBL2332550) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430656 (CHEMBL2332548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430664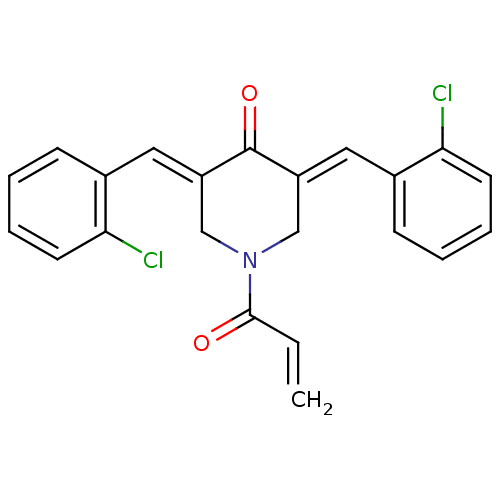 (CHEMBL2332535) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430666 (CHEMBL2332538) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430656 (CHEMBL2332548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430667 (CHEMBL156016) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430655 (CHEMBL2332549) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430671 (CHEMBL2332530) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430660 (CHEMBL454594) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430651 (CHEMBL2332976) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430678 (CHEMBL2332544) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430676 (CHEMBL2332542) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430671 (CHEMBL2332530) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430678 (CHEMBL2332544) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430653 (CHEMBL2332974) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430680 (CHEMBL2332980) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430682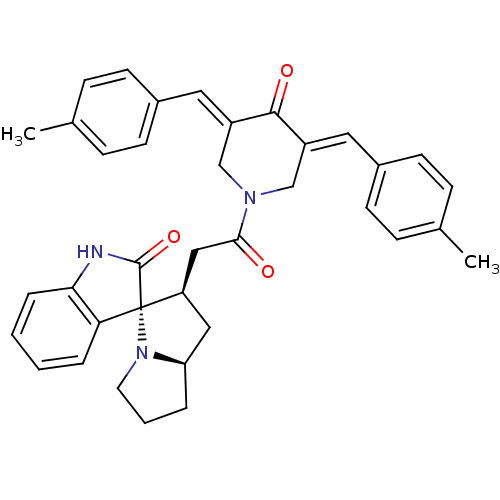 (CHEMBL2332978) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430668 (CHEMBL2332981) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430683 (CHEMBL2332977) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50430659 (CHEMBL152675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine iodide substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50430667 (CHEMBL156016) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Sains Malaysia Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using S-butyrylthiocholine chloride substrate by Ellman's method based spectrophotometry | Bioorg Med Chem 21: 1696-707 (2013) Article DOI: 10.1016/j.bmc.2013.01.066 BindingDB Entry DOI: 10.7270/Q2RJ4KVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 68 total ) | Next | Last >> |