 Found 44 hits with Last Name = 'li' and Initial = 'ct'
Found 44 hits with Last Name = 'li' and Initial = 'ct' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086083

(1-[2-(3,4,5-Trimethoxy-phenyl)-pent-4-enoyl]-piper...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](CC=C)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H47NO11/c1-7-11-29(27-22-34(47-4)37(49-6)35(23-27)48-5)38(43)40-19-9-8-14-30(40)39(44)51-31(26-12-10-13-28(21-26)50-24-36(41)42)17-15-25-16-18-32(45-2)33(20-25)46-3/h7,10,12-13,16,18,20-23,29-31H,1,8-9,11,14-15,17,19,24H2,2-6H3,(H,41,42)/t29-,30-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086090

(1-[2-(3,4,5-Trimethoxy-phenyl)-butyryl]-piperidine...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)OC(CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C38H47NO11/c1-7-28(26-21-33(46-4)36(48-6)34(22-26)47-5)37(42)39-18-9-8-13-29(39)38(43)50-30(25-11-10-12-27(20-25)49-23-35(40)41)16-14-24-15-17-31(44-2)32(19-24)45-3/h10-12,15,17,19-22,28-30H,7-9,13-14,16,18,23H2,1-6H3,(H,40,41)/t28-,29-,30?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086094

(1-[2-(3,4,5-Trimethoxy-phenyl)-pentanoyl]-piperidi...)Show SMILES CCC[C@H](C(=O)N1CCCC[C@H]1C(=O)OC(CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C39H49NO11/c1-7-11-29(27-22-34(47-4)37(49-6)35(23-27)48-5)38(43)40-19-9-8-14-30(40)39(44)51-31(26-12-10-13-28(21-26)50-24-36(41)42)17-15-25-16-18-32(45-2)33(20-25)46-3/h10,12-13,16,18,20-23,29-31H,7-9,11,14-15,17,19,24H2,1-6H3,(H,41,42)/t29-,30-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086077
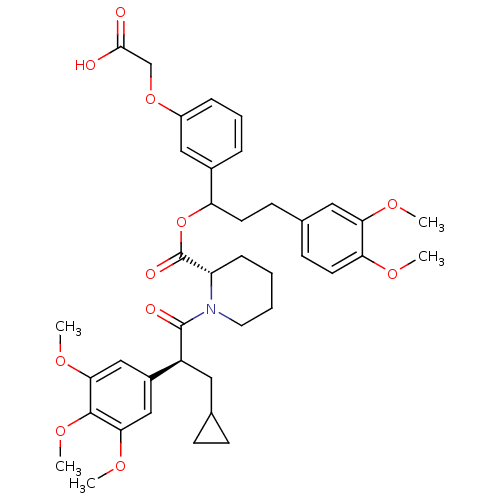
(1-[3-Cyclopropyl-2-(3,4,5-trimethoxy-phenyl)-propi...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](CC2CC2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C40H49NO11/c1-46-33-17-15-26(20-34(33)47-2)14-16-32(27-9-8-10-29(21-27)51-24-37(42)43)52-40(45)31-11-6-7-18-41(31)39(44)30(19-25-12-13-25)28-22-35(48-3)38(50-5)36(23-28)49-4/h8-10,15,17,20-23,25,30-32H,6-7,11-14,16,18-19,24H2,1-5H3,(H,42,43)/t30-,31-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132554

((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-butyryl]-pi...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C36H43NO9/c1-5-28(26-21-31(42-2)34(44-4)32(22-26)43-3)35(40)37-19-10-9-16-29(37)36(41)46-30(18-17-24-12-7-6-8-13-24)25-14-11-15-27(20-25)45-23-33(38)39/h6-8,11-15,20-22,28-30H,5,9-10,16-19,23H2,1-4H3,(H,38,39)/t28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086092

(1-[3-Methyl-2-(3,4,5-trimethoxy-phenyl)-butyryl]-p...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](C(C)C)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H49NO11/c1-24(2)36(27-21-33(47-5)37(49-7)34(22-27)48-6)38(43)40-18-9-8-13-29(40)39(44)51-30(26-11-10-12-28(20-26)50-23-35(41)42)16-14-25-15-17-31(45-3)32(19-25)46-4/h10-12,15,17,19-22,24,29-30,36H,8-9,13-14,16,18,23H2,1-7H3,(H,41,42)/t29-,30?,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132550

((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-propionyl]-...)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](C)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C37H45NO11/c1-23(26-20-32(45-4)35(47-6)33(21-26)46-5)36(41)38-17-8-7-12-28(38)37(42)49-29(25-10-9-11-27(19-25)48-22-34(39)40)15-13-24-14-16-30(43-2)31(18-24)44-3/h9-11,14,16,18-21,23,28-29H,7-8,12-13,15,17,22H2,1-6H3,(H,39,40)/t23-,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132541

((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-butyryl]-pi...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C38H47NO11/c1-7-28(26-21-33(46-4)36(48-6)34(22-26)47-5)37(42)39-18-9-8-13-29(39)38(43)50-30(25-11-10-12-27(20-25)49-23-35(40)41)16-14-24-15-17-31(44-2)32(19-24)45-3/h10-12,15,17,19-22,28-30H,7-9,13-14,16,18,23H2,1-6H3,(H,40,41)/t28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132541

((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-butyryl]-pi...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 |r| Show InChI InChI=1S/C38H47NO11/c1-7-28(26-21-33(46-4)36(48-6)34(22-26)47-5)37(42)39-18-9-8-13-29(39)38(43)50-30(25-11-10-12-27(20-25)49-23-35(40)41)16-14-24-15-17-31(44-2)32(19-24)45-3/h10-12,15,17,19-22,28-30H,7-9,13-14,16,18,23H2,1-6H3,(H,40,41)/t28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132558

((E)-(12S,13R,14S,17R,21S,23S,24R,25R,27R)-17-Ethyl...)Show SMILES CC[C@@H]1\C=C(C)\C[C@H](C)C[C@H](OC)[C@H]2OC([C@H](C)C[C@H]2OC)C(=O)C(=O)N2CCCCC2C(=O)O[C@@H]([C@H](C)[C@@H](O)CC1=O)C(\C)=C\[C@@H]1CC[C@@H](O)[C@@H](C1)OC |c:3| Show InChI InChI=1S/C43H69NO11/c1-10-30-18-24(2)17-25(3)19-36(52-8)41-37(53-9)21-27(5)40(54-41)38(48)42(49)44-16-12-11-13-31(44)43(50)55-39(28(6)33(46)23-34(30)47)26(4)20-29-14-15-32(45)35(22-29)51-7/h18,20,25,27-33,35-37,39-41,45-46H,10-17,19,21-23H2,1-9H3/b24-18+,26-20+/t25-,27+,28+,29-,30+,31?,32+,33-,35+,36-,37+,39+,40?,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086078

(1-(2-Cyclohexyl-2-phenyl-acetyl)-piperidine-2-carb...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@H](C2CCCCC2)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H47NO8/c1-45-34-22-20-27(24-35(34)46-2)19-21-33(30-16-11-17-31(25-30)47-26-36(41)42)48-39(44)32-18-9-10-23-40(32)38(43)37(28-12-5-3-6-13-28)29-14-7-4-8-15-29/h3,5-6,11-13,16-17,20,22,24-25,29,32-33,37H,4,7-10,14-15,18-19,21,23,26H2,1-2H3,(H,41,42)/t32-,33?,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132542
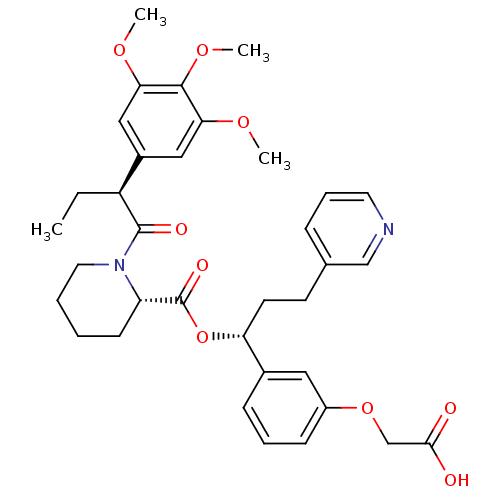
((S)-1-[(S)-2-(3,4,5-Trimethoxy-phenyl)-butyryl]-pi...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1cccnc1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C35H42N2O9/c1-5-27(25-19-30(42-2)33(44-4)31(20-25)43-3)34(40)37-17-7-6-13-28(37)35(41)46-29(15-14-23-10-9-16-36-21-23)24-11-8-12-26(18-24)45-22-32(38)39/h8-12,16,18-21,27-29H,5-7,13-15,17,22H2,1-4H3,(H,38,39)/t27-,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086082
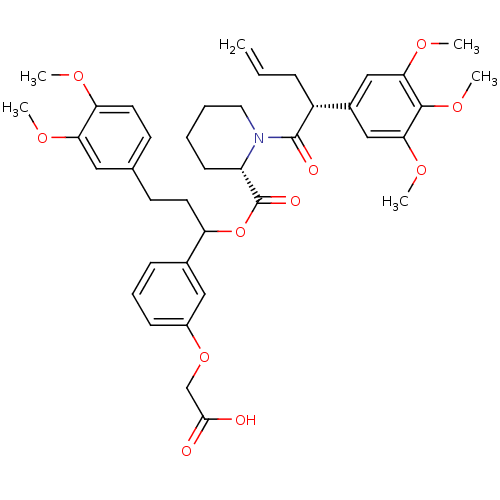
(1-[2-(3,4,5-Trimethoxy-phenyl)-pent-4-enoyl]-piper...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC=C)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H47NO11/c1-7-11-29(27-22-34(47-4)37(49-6)35(23-27)48-5)38(43)40-19-9-8-14-30(40)39(44)51-31(26-12-10-13-28(21-26)50-24-36(41)42)17-15-25-16-18-32(45-2)33(20-25)46-3/h7,10,12-13,16,18,20-23,29-31H,1,8-9,11,14-15,17,19,24H2,2-6H3,(H,41,42)/t29-,30+,31?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086087
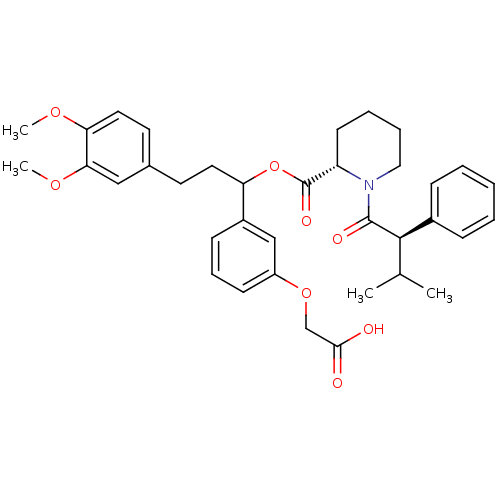
(1-(3-Methyl-2-phenyl-butyryl)-piperidine-2-carboxy...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@H](C(C)C)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C36H43NO8/c1-24(2)34(26-11-6-5-7-12-26)35(40)37-20-9-8-15-29(37)36(41)45-30(27-13-10-14-28(22-27)44-23-33(38)39)18-16-25-17-19-31(42-3)32(21-25)43-4/h5-7,10-14,17,19,21-22,24,29-30,34H,8-9,15-16,18,20,23H2,1-4H3,(H,38,39)/t29-,30?,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132560
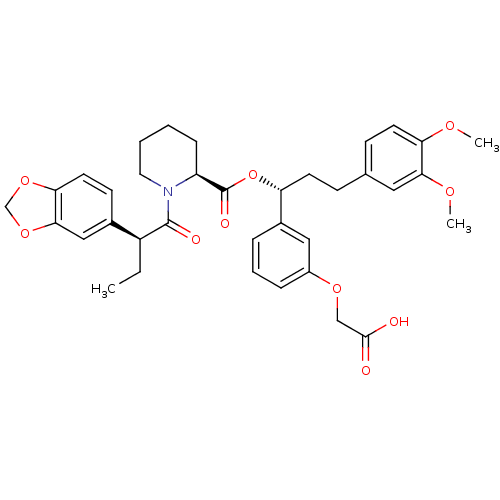
((S)-1-((S)-2-Benzo[1,3]dioxol-5-yl-butyryl)-piperi...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1ccc2OCOc2c1 Show InChI InChI=1S/C36H41NO10/c1-4-27(24-13-16-31-33(20-24)46-22-45-31)35(40)37-17-6-5-10-28(37)36(41)47-29(25-8-7-9-26(19-25)44-21-34(38)39)14-11-23-12-15-30(42-2)32(18-23)43-3/h7-9,12-13,15-16,18-20,27-29H,4-6,10-11,14,17,21-22H2,1-3H3,(H,38,39)/t27-,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086085
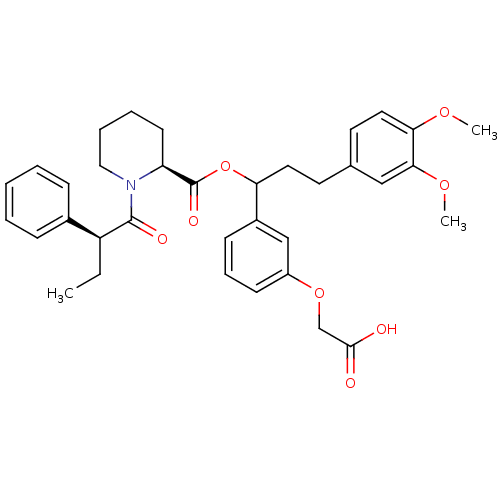
(1-(2-Phenyl-butyryl)-piperidine-2-carboxylic acid ...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)OC(CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1ccccc1 Show InChI InChI=1S/C35H41NO8/c1-4-28(25-11-6-5-7-12-25)34(39)36-20-9-8-15-29(36)35(40)44-30(26-13-10-14-27(22-26)43-23-33(37)38)18-16-24-17-19-31(41-2)32(21-24)42-3/h5-7,10-14,17,19,21-22,28-30H,4,8-9,15-16,18,20,23H2,1-3H3,(H,37,38)/t28?,29-,30?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132544
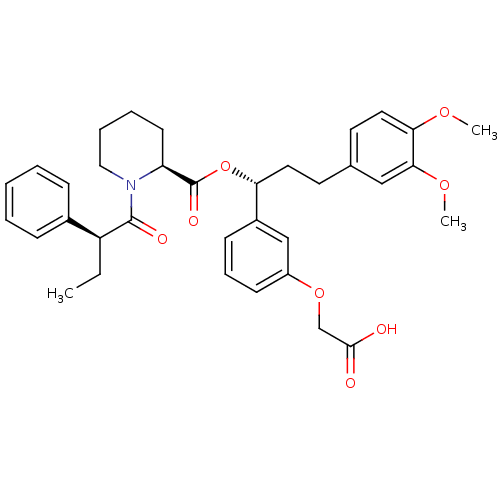
((S)-1-((S)-2-Phenyl-butyryl)-piperidine-2-carboxyl...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1ccccc1 Show InChI InChI=1S/C35H41NO8/c1-4-28(25-11-6-5-7-12-25)34(39)36-20-9-8-15-29(36)35(40)44-30(26-13-10-14-27(22-26)43-23-33(37)38)18-16-24-17-19-31(41-2)32(21-24)42-3/h5-7,10-14,17,19,21-22,28-30H,4,8-9,15-16,18,20,23H2,1-3H3,(H,37,38)/t28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132544

((S)-1-((S)-2-Phenyl-butyryl)-piperidine-2-carboxyl...)Show SMILES CC[C@H](C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1ccccc1 Show InChI InChI=1S/C35H41NO8/c1-4-28(25-11-6-5-7-12-25)34(39)36-20-9-8-15-29(36)35(40)44-30(26-13-10-14-27(22-26)43-23-33(37)38)18-16-24-17-19-31(41-2)32(21-24)42-3/h5-7,10-14,17,19,21-22,28-30H,4,8-9,15-16,18,20,23H2,1-3H3,(H,37,38)/t28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086075
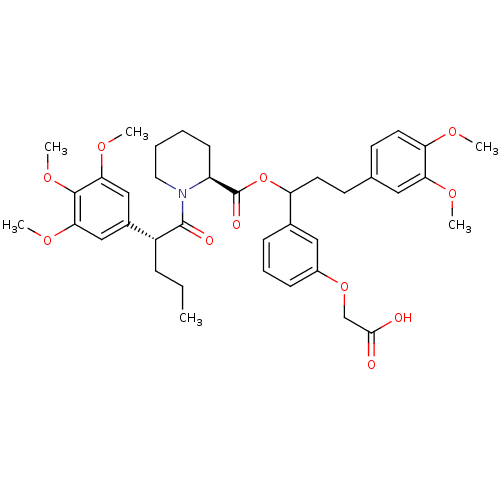
(1-[2-(3,4,5-Trimethoxy-phenyl)-pentanoyl]-piperidi...)Show SMILES CCC[C@@H](C(=O)N1CCCC[C@H]1C(=O)OC(CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C39H49NO11/c1-7-11-29(27-22-34(47-4)37(49-6)35(23-27)48-5)38(43)40-19-9-8-14-30(40)39(44)51-31(26-12-10-13-28(21-26)50-24-36(41)42)17-15-25-16-18-32(45-2)33(20-25)46-3/h10,12-13,16,18,20-23,29-31H,7-9,11,14-15,17,19,24H2,1-6H3,(H,41,42)/t29-,30+,31?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132556
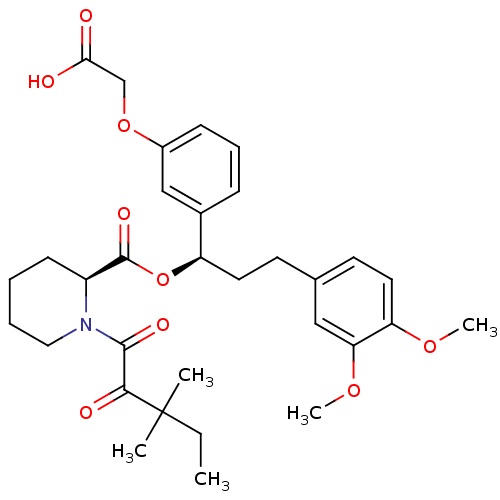
((S)-1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1 Show InChI InChI=1S/C32H41NO9/c1-6-32(2,3)29(36)30(37)33-17-8-7-12-24(33)31(38)42-25(22-10-9-11-23(19-22)41-20-28(34)35)15-13-21-14-16-26(39-4)27(18-21)40-5/h9-11,14,16,18-19,24-25H,6-8,12-13,15,17,20H2,1-5H3,(H,34,35)/t24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132551
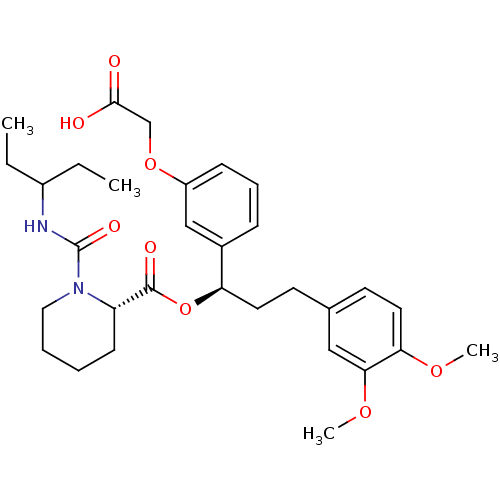
(1-(1-Ethyl-propylcarbamoyl)-piperidine-2-carboxyli...)Show SMILES CCC(CC)NC(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1 Show InChI InChI=1S/C31H42N2O8/c1-5-23(6-2)32-31(37)33-17-8-7-12-25(33)30(36)41-26(22-10-9-11-24(19-22)40-20-29(34)35)15-13-21-14-16-27(38-3)28(18-21)39-4/h9-11,14,16,18-19,23,25-26H,5-8,12-13,15,17,20H2,1-4H3,(H,32,37)(H,34,35)/t25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086086
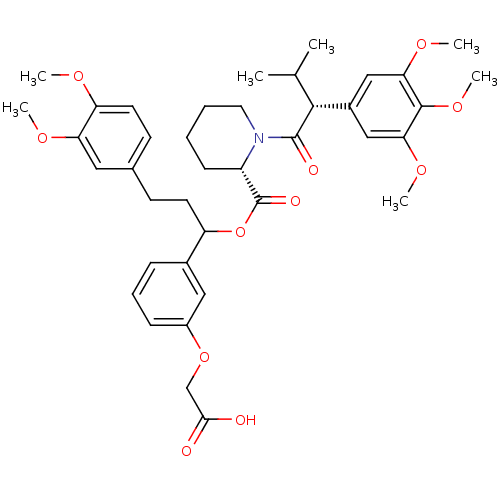
(1-[3-Methyl-2-(3,4,5-trimethoxy-phenyl)-butyryl]-p...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@H](C(C)C)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H49NO11/c1-24(2)36(27-21-33(47-5)37(49-7)34(22-27)48-6)38(43)40-18-9-8-13-29(40)39(44)51-30(26-11-10-12-28(20-26)50-23-35(41)42)16-14-25-15-17-31(45-3)32(19-25)46-4/h10-12,15,17,19-22,24,29-30,36H,8-9,13-14,16,18,23H2,1-7H3,(H,41,42)/t29-,30?,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086079
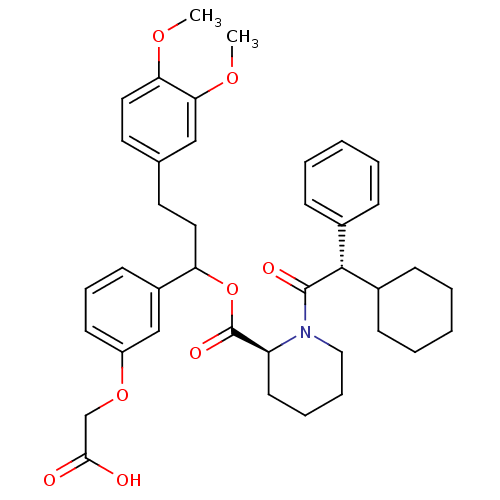
(1-(2-Cyclohexyl-2-phenyl-acetyl)-piperidine-2-carb...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](C2CCCCC2)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H47NO8/c1-45-34-22-20-27(24-35(34)46-2)19-21-33(30-16-11-17-31(25-30)47-26-36(41)42)48-39(44)32-18-9-10-23-40(32)38(43)37(28-12-5-3-6-13-28)29-14-7-4-8-15-29/h3,5-6,11-13,16-17,20,22,24-25,29,32-33,37H,4,7-10,14-15,18-19,21,23,26H2,1-2H3,(H,41,42)/t32-,33?,37+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086080
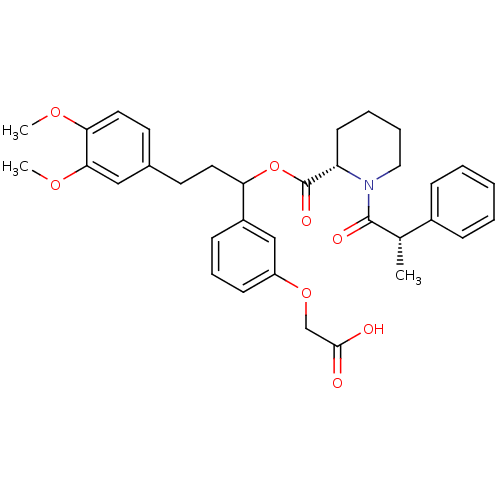
(1-(2-Phenyl-propionyl)-piperidine-2-carboxylic aci...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](C)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C34H39NO8/c1-23(25-10-5-4-6-11-25)33(38)35-19-8-7-14-28(35)34(39)43-29(26-12-9-13-27(21-26)42-22-32(36)37)17-15-24-16-18-30(40-2)31(20-24)41-3/h4-6,9-13,16,18,20-21,23,28-29H,7-8,14-15,17,19,22H2,1-3H3,(H,36,37)/t23-,28-,29?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086089
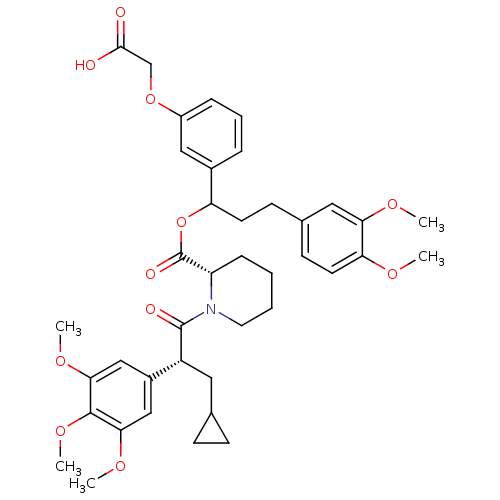
(1-[3-Cyclopropyl-2-(3,4,5-trimethoxy-phenyl)-propi...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@H](CC2CC2)c2cc(OC)c(OC)c(OC)c2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C40H49NO11/c1-46-33-17-15-26(20-34(33)47-2)14-16-32(27-9-8-10-29(21-27)51-24-37(42)43)52-40(45)31-11-6-7-18-41(31)39(44)30(19-25-12-13-25)28-22-35(48-3)38(50-5)36(23-28)49-4/h8-10,15,17,20-23,25,30-32H,6-7,11-14,16,18-19,24H2,1-5H3,(H,42,43)/t30-,31+,32?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086084
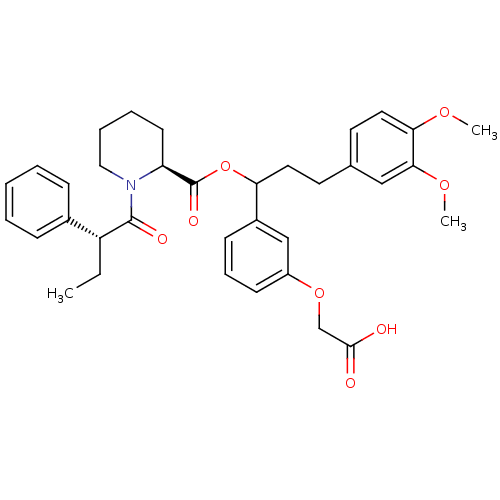
(1-(2-Phenyl-butyryl)-piperidine-2-carboxylic acid ...)Show SMILES CC[C@@H](C(=O)N1CCCC[C@H]1C(=O)OC(CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1ccccc1 Show InChI InChI=1S/C35H41NO8/c1-4-28(25-11-6-5-7-12-25)34(39)36-20-9-8-15-29(36)35(40)44-30(26-13-10-14-27(22-26)43-23-33(37)38)18-16-24-17-19-31(41-2)32(21-24)42-3/h5-7,10-14,17,19,21-22,28-30H,4,8-9,15-16,18,20,23H2,1-3H3,(H,37,38)/t28-,29+,30?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086081
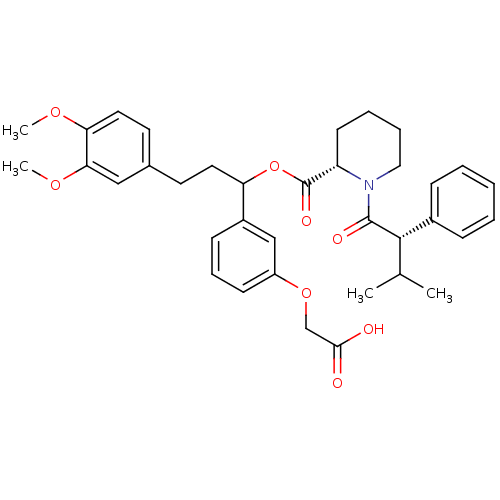
(1-(3-Methyl-2-phenyl-butyryl)-piperidine-2-carboxy...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@@H](C(C)C)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C36H43NO8/c1-24(2)34(26-11-6-5-7-12-26)35(40)37-20-9-8-15-29(37)36(41)45-30(27-13-10-14-28(22-27)44-23-33(38)39)18-16-25-17-19-31(42-3)32(21-25)43-4/h5-7,10-14,17,19,21-22,24,29-30,34H,8-9,15-16,18,20,23H2,1-4H3,(H,38,39)/t29-,30?,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086093
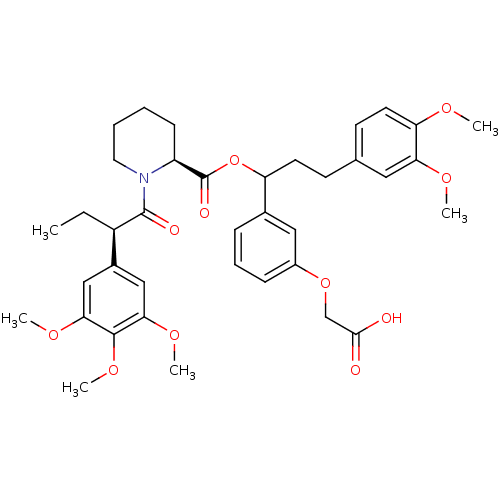
(1-[2-(3,4,5-Trimethoxy-phenyl)-butyryl]-piperidine...)Show SMILES CC[C@@H](C(=O)N1CCCC[C@H]1C(=O)OC(CCc1ccc(OC)c(OC)c1)c1cccc(OCC(O)=O)c1)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C38H47NO11/c1-7-28(26-21-33(46-4)36(48-6)34(22-26)47-5)37(42)39-18-9-8-13-29(39)38(43)50-30(25-11-10-12-27(20-25)49-23-35(40)41)16-14-24-15-17-31(44-2)32(19-24)45-3/h10-12,15,17,19-22,28-30H,7-9,13-14,16,18,23H2,1-6H3,(H,40,41)/t28-,29+,30?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086076
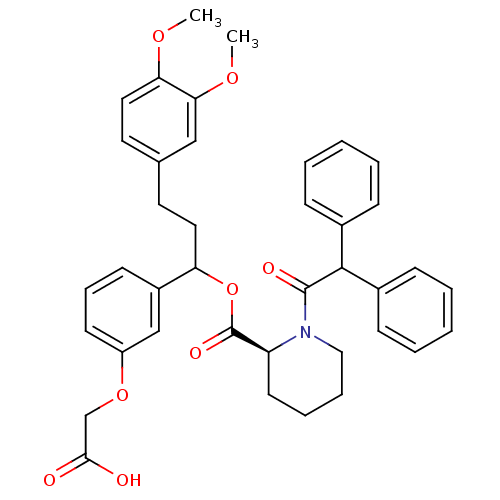
(1-Diphenylacetyl-piperidine-2-carboxylic acid 1-(3...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)C(c2ccccc2)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C39H41NO8/c1-45-34-22-20-27(24-35(34)46-2)19-21-33(30-16-11-17-31(25-30)47-26-36(41)42)48-39(44)32-18-9-10-23-40(32)38(43)37(28-12-5-3-6-13-28)29-14-7-4-8-15-29/h3-8,11-17,20,22,24-25,32-33,37H,9-10,18-19,21,23,26H2,1-2H3,(H,41,42)/t32-,33?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50035703
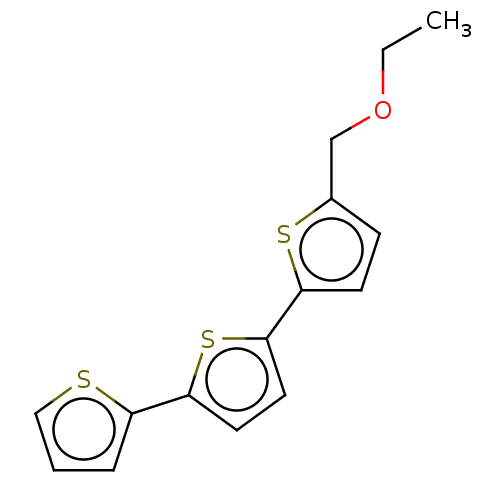
(CHEMBL3343903)Show InChI InChI=1S/C15H14OS3/c1-2-16-10-11-5-6-14(18-11)15-8-7-13(19-15)12-4-3-9-17-12/h3-9H,2,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human plasma using Gly-Pro-AMC substrate incubated for 20 mins by fluorometry |
Bioorg Med Chem 22: 6515-22 (2015)
Article DOI: 10.1016/j.bmc.2014.06.051
BindingDB Entry DOI: 10.7270/Q21Z4611 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50086091
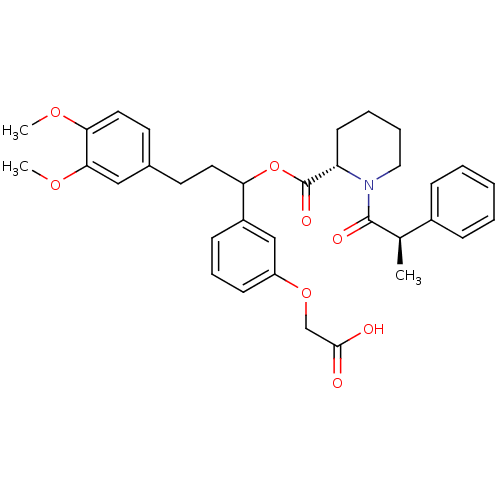
(1-(2-Phenyl-propionyl)-piperidine-2-carboxylic aci...)Show SMILES COc1ccc(CCC(OC(=O)[C@@H]2CCCCN2C(=O)[C@H](C)c2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C34H39NO8/c1-23(25-10-5-4-6-11-25)33(38)35-19-8-7-14-28(35)34(39)43-29(26-12-9-13-27(21-26)42-22-32(36)37)17-15-24-16-18-30(40-2)31(20-24)41-3/h4-6,9-13,16,18,20-21,23,28-29H,7-8,14-15,17,19,22H2,1-3H3,(H,36,37)/t23-,28+,29?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50035706
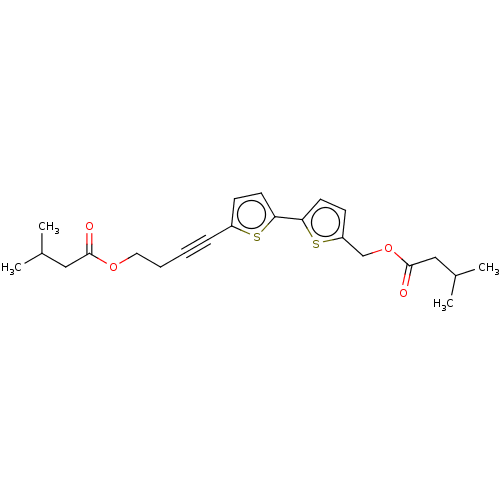
(CHEMBL3343901)Show SMILES CC(C)CC(=O)OCCC#Cc1ccc(s1)-c1ccc(COC(=O)CC(C)C)s1 Show InChI InChI=1S/C23H28O4S2/c1-16(2)13-22(24)26-12-6-5-7-18-8-10-20(28-18)21-11-9-19(29-21)15-27-23(25)14-17(3)4/h8-11,16-17H,6,12-15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human plasma using Gly-Pro-AMC substrate incubated for 20 mins by fluorometry |
Bioorg Med Chem 22: 6515-22 (2015)
Article DOI: 10.1016/j.bmc.2014.06.051
BindingDB Entry DOI: 10.7270/Q21Z4611 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50035702
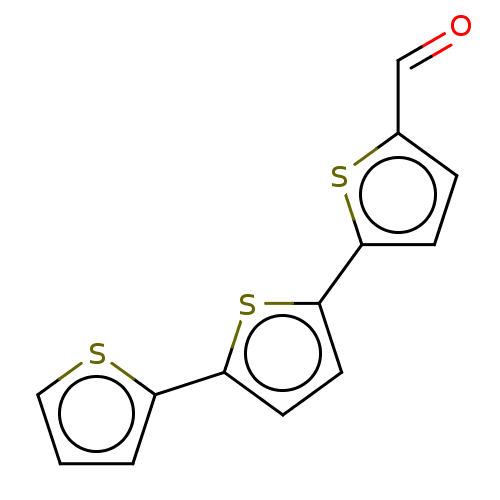
(CHEMBL91933)Show InChI InChI=1S/C13H8OS3/c14-8-9-3-4-12(16-9)13-6-5-11(17-13)10-2-1-7-15-10/h1-8H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human plasma using Gly-Pro-AMC substrate incubated for 20 mins by fluorometry |
Bioorg Med Chem 22: 6515-22 (2015)
Article DOI: 10.1016/j.bmc.2014.06.051
BindingDB Entry DOI: 10.7270/Q21Z4611 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132547
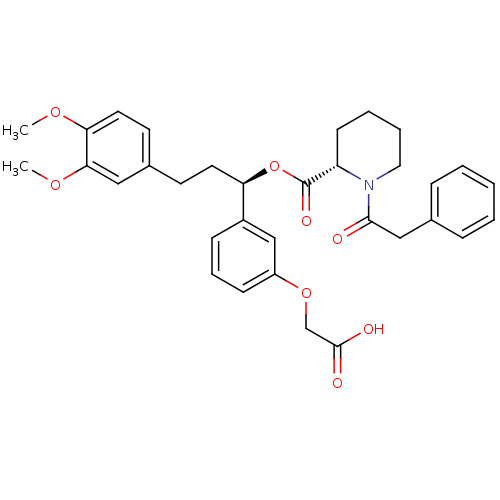
(1-Phenylacetyl-piperidine-2-carboxylic acid 1-(3-c...)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)Cc2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C33H37NO8/c1-39-29-17-15-24(19-30(29)40-2)14-16-28(25-11-8-12-26(21-25)41-22-32(36)37)42-33(38)27-13-6-7-18-34(27)31(35)20-23-9-4-3-5-10-23/h3-5,8-12,15,17,19,21,27-28H,6-7,13-14,16,18,20,22H2,1-2H3,(H,36,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132547

(1-Phenylacetyl-piperidine-2-carboxylic acid 1-(3-c...)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)Cc2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C33H37NO8/c1-39-29-17-15-24(19-30(29)40-2)14-16-28(25-11-8-12-26(21-25)41-22-32(36)37)42-33(38)27-13-6-7-18-34(27)31(35)20-23-9-4-3-5-10-23/h3-5,8-12,15,17,19,21,27-28H,6-7,13-14,16,18,20,22H2,1-2H3,(H,36,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. |
J Med Chem 43: 1135-42 (2000)
BindingDB Entry DOI: 10.7270/Q2057F45 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50132547

(1-Phenylacetyl-piperidine-2-carboxylic acid 1-(3-c...)Show SMILES COc1ccc(CC[C@@H](OC(=O)[C@@H]2CCCCN2C(=O)Cc2ccccc2)c2cccc(OCC(O)=O)c2)cc1OC Show InChI InChI=1S/C33H37NO8/c1-39-29-17-15-24(19-30(29)40-2)14-16-28(25-11-8-12-26(21-25)41-22-32(36)37)42-33(38)27-13-6-7-18-34(27)31(35)20-23-9-4-3-5-10-23/h3-5,8-12,15,17,19,21,27-28H,6-7,13-14,16,18,20,22H2,1-2H3,(H,36,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of binding to Phe36Val (F36V) mutant of FK506 binding protein 12 |
Bioorg Med Chem Lett 13: 3181-4 (2003)
BindingDB Entry DOI: 10.7270/Q2BV7G0S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50035704
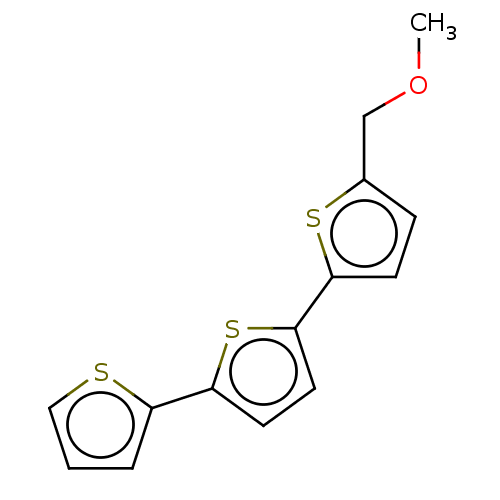
(CHEMBL3343902)Show InChI InChI=1S/C14H12OS3/c1-15-9-10-4-5-13(17-10)14-7-6-12(18-14)11-3-2-8-16-11/h2-8H,9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human plasma using Gly-Pro-AMC substrate incubated for 20 mins by fluorometry |
Bioorg Med Chem 22: 6515-22 (2015)
Article DOI: 10.1016/j.bmc.2014.06.051
BindingDB Entry DOI: 10.7270/Q21Z4611 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50229666
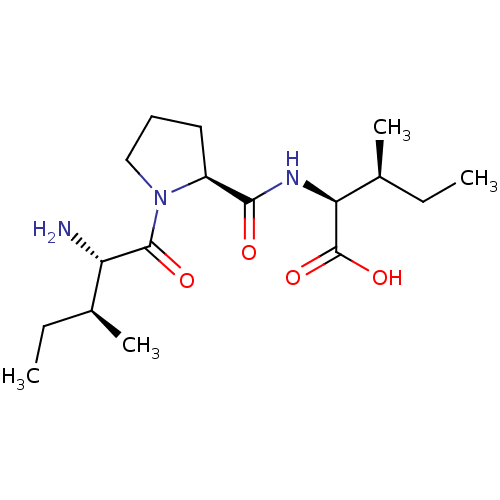
((2S,3S)-2-((S)-1-((2S,3S)-2-amino-3-methylpentanoy...)Show SMILES CC[C@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(O)=O Show InChI InChI=1S/C17H31N3O4/c1-5-10(3)13(18)16(22)20-9-7-8-12(20)15(21)19-14(17(23)24)11(4)6-2/h10-14H,5-9,18H2,1-4H3,(H,19,21)(H,23,24)/t10-,11-,12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human plasma using Gly-Pro-AMC substrate incubated for 20 mins by fluorometry |
Bioorg Med Chem 22: 6515-22 (2015)
Article DOI: 10.1016/j.bmc.2014.06.051
BindingDB Entry DOI: 10.7270/Q21Z4611 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50035708
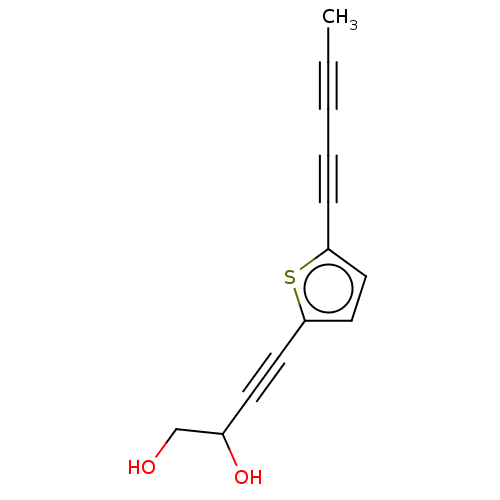
(CHEMBL3343899)Show InChI InChI=1S/C13H10O2S/c1-2-3-4-5-12-8-9-13(16-12)7-6-11(15)10-14/h8-9,11,14-15H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human plasma using Gly-Pro-AMC substrate incubated for 20 mins by fluorometry |
Bioorg Med Chem 22: 6515-22 (2015)
Article DOI: 10.1016/j.bmc.2014.06.051
BindingDB Entry DOI: 10.7270/Q21Z4611 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50035707
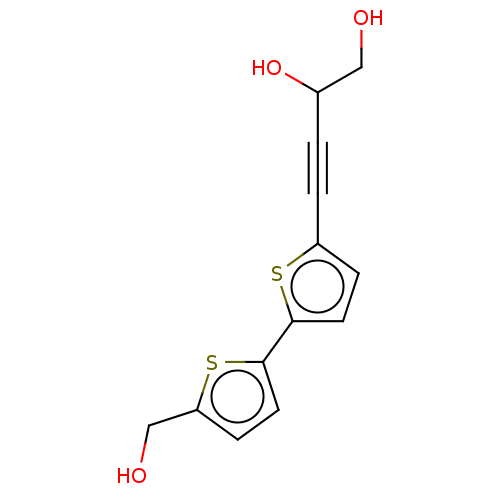
(CHEMBL3343900)Show InChI InChI=1S/C13H12O3S2/c14-7-9(16)1-2-10-3-5-12(17-10)13-6-4-11(8-15)18-13/h3-6,9,14-16H,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human plasma using Gly-Pro-AMC substrate incubated for 20 mins by fluorometry |
Bioorg Med Chem 22: 6515-22 (2015)
Article DOI: 10.1016/j.bmc.2014.06.051
BindingDB Entry DOI: 10.7270/Q21Z4611 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50035705
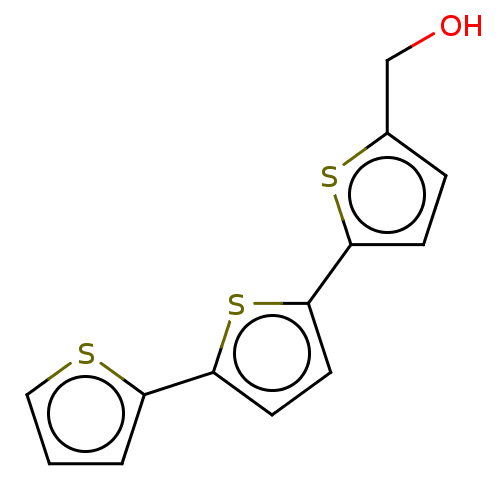
(CHEMBL90170)Show InChI InChI=1S/C13H10OS3/c14-8-9-3-4-12(16-9)13-6-5-11(17-13)10-2-1-7-15-10/h1-7,14H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human plasma using Gly-Pro-AMC substrate incubated for 20 mins by fluorometry |
Bioorg Med Chem 22: 6515-22 (2015)
Article DOI: 10.1016/j.bmc.2014.06.051
BindingDB Entry DOI: 10.7270/Q21Z4611 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50004558
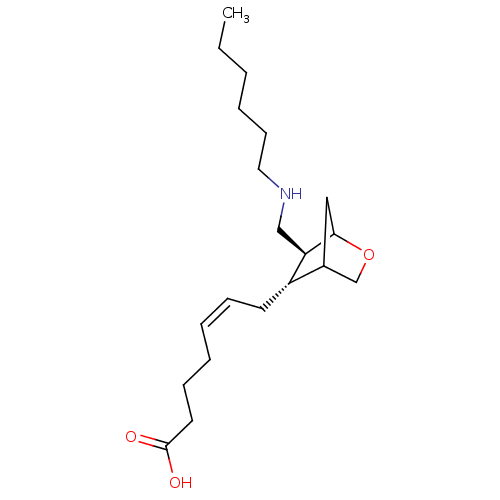
(7-(6-Hexylaminomethyl-2-oxa-bicyclo[2.2.1]hept-5-y...)Show SMILES CCCCCCNC[C@H]1C2CC(CO2)[C@@H]1C\C=C/CCCC(O)=O |TLB:15:14:10:13.12,THB:7:8:10:13.12| Show InChI InChI=1S/C20H35NO3/c1-2-3-4-9-12-21-14-18-17(16-13-19(18)24-15-16)10-7-5-6-8-11-20(22)23/h5,7,16-19,21H,2-4,6,8-15H2,1H3,(H,22,23)/b7-5-/t16?,17-,18+,19?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Compound was evaluated for the competitive binding of [3H]-U-46,619 to washed washed human platelets at the Thromboxane A2 receptor |
J Med Chem 35: 3033-9 (1992)
BindingDB Entry DOI: 10.7270/Q2C53JT8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50035700
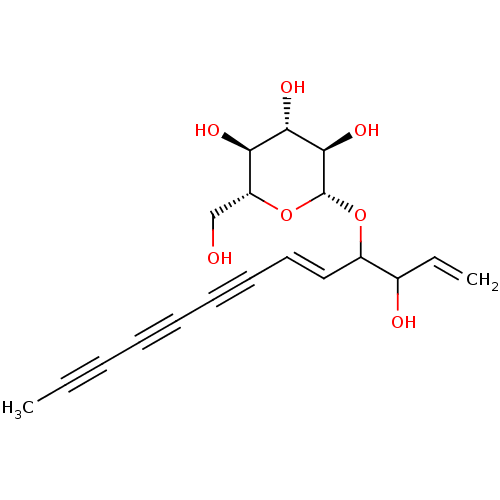
(CHEMBL3343905)Show SMILES CC#CC#CC#C\C=C\C(O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)C(O)C=C |r| Show InChI InChI=1S/C19H22O7/c1-3-5-6-7-8-9-10-11-14(13(21)4-2)25-19-18(24)17(23)16(22)15(12-20)26-19/h4,10-11,13-24H,2,12H2,1H3/b11-10+/t13?,14?,15-,16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human plasma using Gly-Pro-AMC substrate incubated for 20 mins by fluorometry |
Bioorg Med Chem 22: 6515-22 (2015)
Article DOI: 10.1016/j.bmc.2014.06.051
BindingDB Entry DOI: 10.7270/Q21Z4611 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50035701
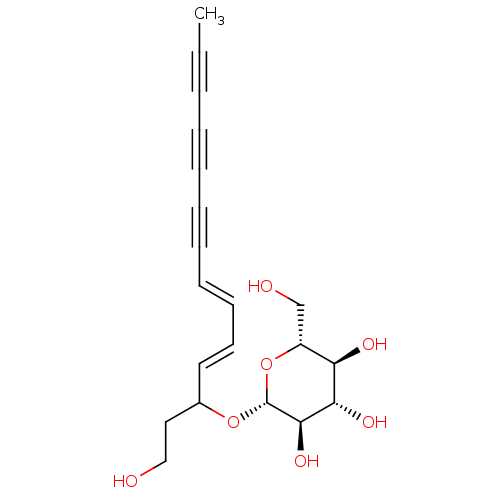
(CHEMBL3343904)Show SMILES CC#CC#CC#C\C=C\C=C\C(CCO)O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H24O7/c1-2-3-4-5-6-7-8-9-10-11-15(12-13-21)26-20-19(25)18(24)17(23)16(14-22)27-20/h8-11,15-25H,12-14H2,1H3/b9-8+,11-10+/t15?,16-,17-,18+,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human plasma using Gly-Pro-AMC substrate incubated for 20 mins by fluorometry |
Bioorg Med Chem 22: 6515-22 (2015)
Article DOI: 10.1016/j.bmc.2014.06.051
BindingDB Entry DOI: 10.7270/Q21Z4611 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data