

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of squalene synthase in rat liver. | Bioorg Med Chem Lett 3: 2029-2034 (1993) Article DOI: 10.1016/S0960-894X(01)81008-8 BindingDB Entry DOI: 10.7270/Q22J6BSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against squalene synthase in rat liver squalene synthase (RLSS) enzyme assay | Bioorg Med Chem Lett 4: 1591-1594 (1994) Article DOI: 10.1016/S0960-894X(01)80572-2 BindingDB Entry DOI: 10.7270/Q2TB16T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Ability to displace [3H]-17-beta-estradiol from Estrogen receptor alpha by scintillation proximity assay. | Bioorg Med Chem Lett 11: 1939-42 (2001) BindingDB Entry DOI: 10.7270/Q2ZK5FZ0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of Aurora B ATP binding site | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of human Aurora B ATP binding site by rapid dilution method | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50469353 (CHEMBL4283353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNC Eshelman School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of MERTK (unknown origin) using 5'-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by MCE assay | J Med Chem 61: 10242-10254 (2018) Article DOI: 10.1021/acs.jmedchem.8b01229 BindingDB Entry DOI: 10.7270/Q2K076ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of AURA | J Med Chem 51: 7898-914 (2008) Article DOI: 10.1021/jm8011036 BindingDB Entry DOI: 10.7270/Q2WS8T4C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Aurora A | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of human Aurora C ATP binding site | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of Aurora B ATP binding site | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Ability to displace [3H]-17-beta-estradiol from Estrogen receptor alpha by scintillation proximity assay. | Bioorg Med Chem Lett 11: 1939-42 (2001) BindingDB Entry DOI: 10.7270/Q2ZK5FZ0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-G-associated kinase (Homo sapiens (Human)) | BDBM50524284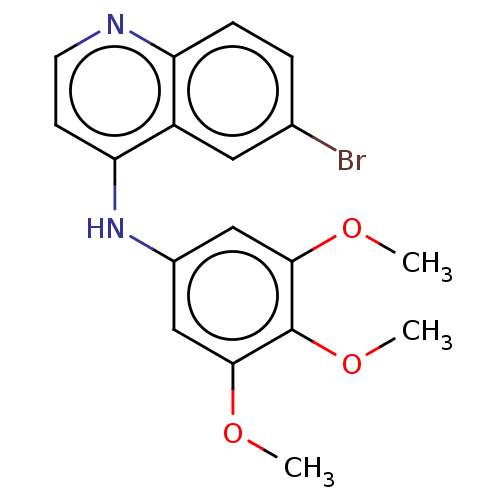 (CHEMBL4443342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe University Curated by ChEMBL | Assay Description Binding affinity to C-terminal AVI-tagged GAK (unknown origin) (12 to 347 residues) expressed in Escherichia coli after 1.5 hrs by TR-FRET assay | J Med Chem 62: 2830-2836 (2019) Article DOI: 10.1021/acs.jmedchem.8b01213 BindingDB Entry DOI: 10.7270/Q28919C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Ability to displace [3H]-17-beta-estradiol from Estrogen receptor beta by scintillation proximity assay. | Bioorg Med Chem Lett 11: 1939-42 (2001) BindingDB Entry DOI: 10.7270/Q2ZK5FZ0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-G-associated kinase (Homo sapiens (Human)) | BDBM50537138 (CHEMBL4531690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe University Curated by ChEMBL | Assay Description Binding affinity to C-terminal AVI-tagged GAK (unknown origin) (12 to 347 residues) expressed in Escherichia coli after 1.5 hrs by TR-FRET assay | J Med Chem 62: 2830-2836 (2019) Article DOI: 10.1021/acs.jmedchem.8b01213 BindingDB Entry DOI: 10.7270/Q28919C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM50469353 (CHEMBL4283353) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNC Eshelman School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of TYRO3 (unknown origin) using 5'-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by MCE assay | J Med Chem 61: 10242-10254 (2018) Article DOI: 10.1021/acs.jmedchem.8b01229 BindingDB Entry DOI: 10.7270/Q2K076ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of AURC | J Med Chem 51: 7898-914 (2008) Article DOI: 10.1021/jm8011036 BindingDB Entry DOI: 10.7270/Q2WS8T4C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of Aurora C ATP binding site | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50230828 (5-(4-fluorophenyl)-2-ureidothiophene-3-carboxamide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin) | J Med Chem 51: 7898-914 (2008) Article DOI: 10.1021/jm8011036 BindingDB Entry DOI: 10.7270/Q2WS8T4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50072588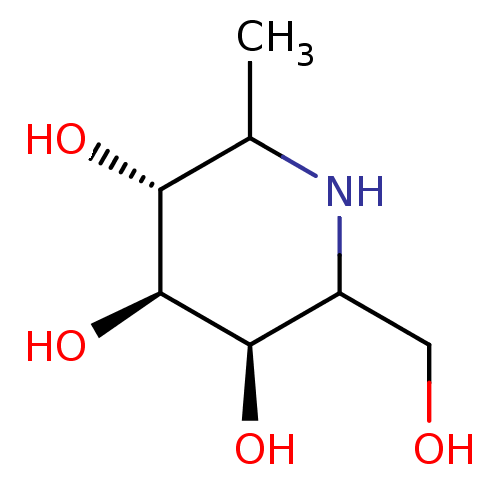 ((2S,3R,4R,5R,6S)-2-Hydroxymethyl-6-methyl-piperidi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibitory activity against alpha-L-fucosidase from bovine epididymus (sigma) | Bioorg Med Chem Lett 3: 2533-2536 (1993) Article DOI: 10.1016/S0960-894X(01)80711-3 BindingDB Entry DOI: 10.7270/Q2BG2PGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50511373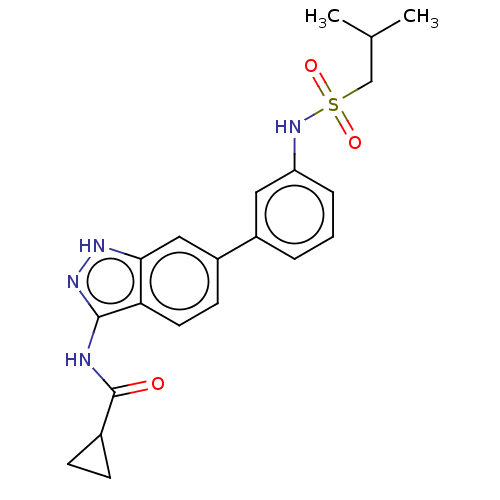 (CHEMBL4571548) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065258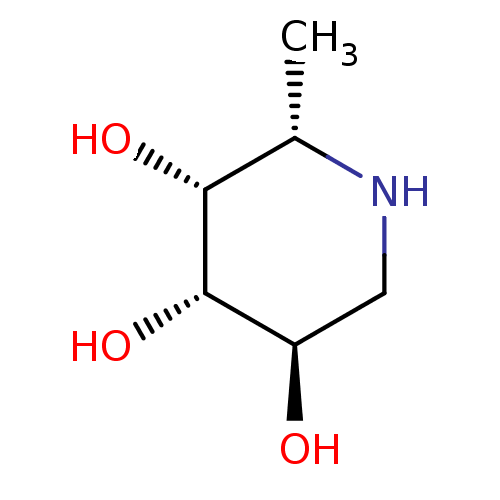 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibitory activity against alpha-L-fucosidase from bovine epididymus (sigma) | Bioorg Med Chem Lett 3: 2533-2536 (1993) Article DOI: 10.1016/S0960-894X(01)80711-3 BindingDB Entry DOI: 10.7270/Q2BG2PGV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50511385 (CHEMBL4534959) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50511383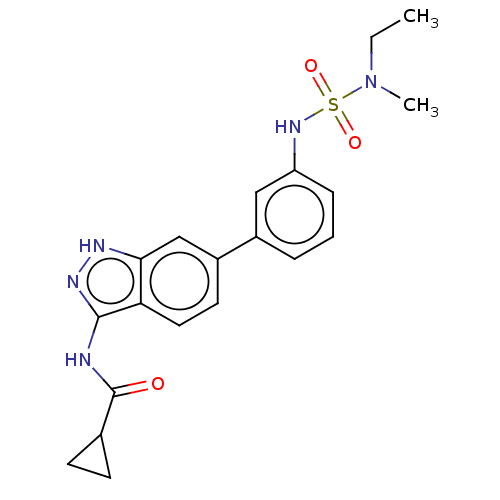 (CHEMBL4531680) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50511376 (CHEMBL4516665) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50511400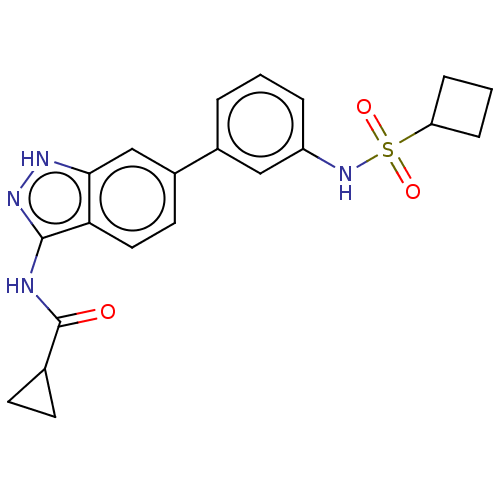 (CHEMBL4535040) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50511397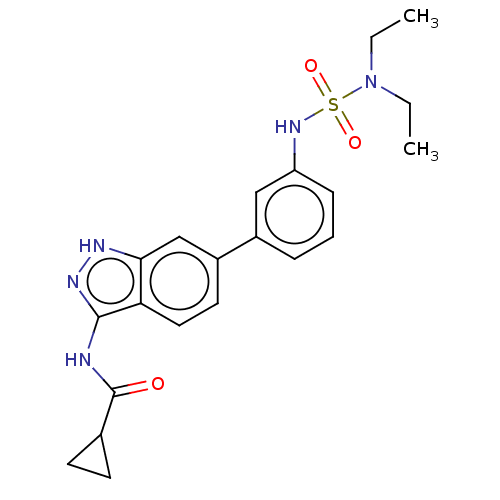 (CHEMBL4452939) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065258 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibitory activity against alpha-L-fucosidase from solubilised human neutrophils | Bioorg Med Chem Lett 3: 2533-2536 (1993) Article DOI: 10.1016/S0960-894X(01)80711-3 BindingDB Entry DOI: 10.7270/Q2BG2PGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50511380 (CHEMBL4452360) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Ability to displace [3H]-17-beta-estradiol from Estrogen receptor beta by scintillation proximity assay. | Bioorg Med Chem Lett 11: 1939-42 (2001) BindingDB Entry DOI: 10.7270/Q2ZK5FZ0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50072588 ((2S,3R,4R,5R,6S)-2-Hydroxymethyl-6-methyl-piperidi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibitory activity against alpha-L-fucosidase from solubilised human neutrophils | Bioorg Med Chem Lett 3: 2533-2536 (1993) Article DOI: 10.1016/S0960-894X(01)80711-3 BindingDB Entry DOI: 10.7270/Q2BG2PGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peripheral plasma membrane protein CASK (Homo sapiens (Human)) | BDBM50574225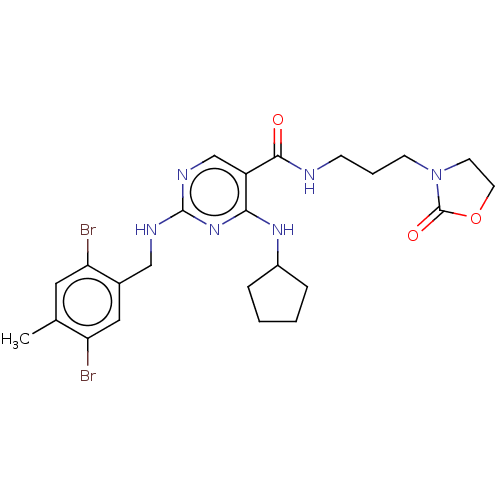 (CHEMBL4850857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of tracer K5 binding to NanoLuc-fused CASK (unknown origin) expressed in HEK293T cells measured after 2 hrs by NanoBRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00845 BindingDB Entry DOI: 10.7270/Q2WH2TSV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50511394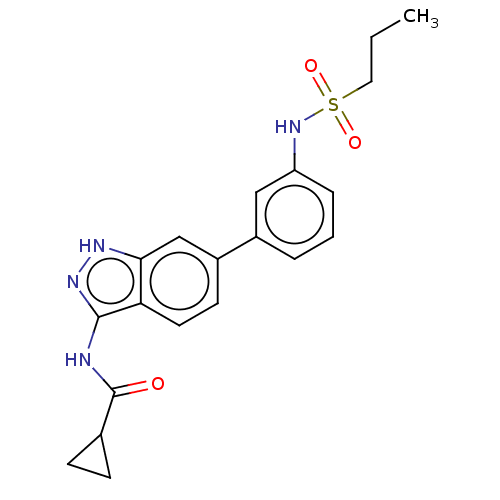 (CHEMBL4552310) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BMP-2-inducible protein kinase (Homo sapiens (Human)) | BDBM50511376 (CHEMBL4516665) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50161646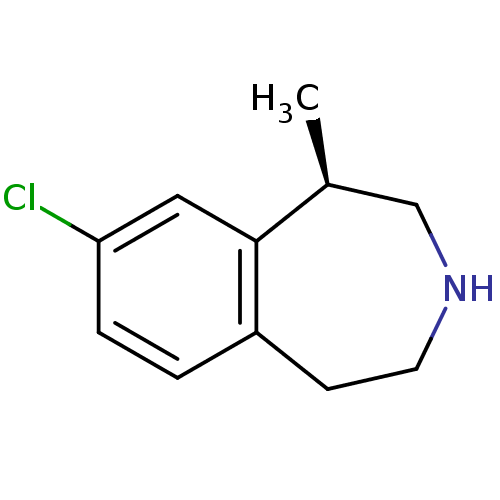 ((1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human recombinant 5HT2C receptor expressed in HEK293 cells by scintillation counting | Bioorg Med Chem Lett 21: 2715-20 (2011) Article DOI: 10.1016/j.bmcl.2010.11.120 BindingDB Entry DOI: 10.7270/Q2HD7VZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peripheral plasma membrane protein CASK (Homo sapiens (Human)) | BDBM50574224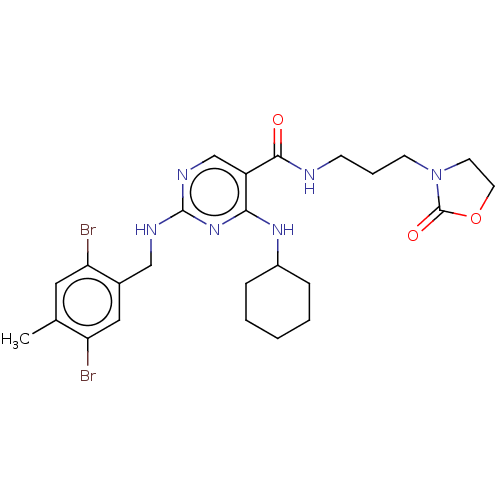 (CHEMBL4876584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of tracer K5 binding to NanoLuc-fused CASK (unknown origin) expressed in HEK293T cells measured after 2 hrs by NanoBRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00845 BindingDB Entry DOI: 10.7270/Q2WH2TSV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peripheral plasma membrane protein CASK (Homo sapiens (Human)) | BDBM50425829 (CHEMBL2312304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of tracer K5 binding to NanoLuc-fused CASK (unknown origin) expressed in HEK293T cells measured after 2 hrs by NanoBRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00845 BindingDB Entry DOI: 10.7270/Q2WH2TSV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50469353 (CHEMBL4283353) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNC Eshelman School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of FLT3 (unknown origin) using 5'-FAM-KKKKEEIYFFF-CONH2 as substrate after 180 mins by MCE assay | J Med Chem 61: 10242-10254 (2018) Article DOI: 10.1021/acs.jmedchem.8b01229 BindingDB Entry DOI: 10.7270/Q2K076ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BMP-2-inducible protein kinase (Homo sapiens (Human)) | BDBM50511400 (CHEMBL4535040) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BMP-2-inducible protein kinase (Homo sapiens (Human)) | BDBM50511385 (CHEMBL4534959) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| BMP-2-inducible protein kinase (Homo sapiens (Human)) | BDBM50511397 (CHEMBL4452939) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BMP-2-inducible protein kinase (Homo sapiens (Human)) | BDBM50511373 (CHEMBL4571548) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of AURB | J Med Chem 51: 7898-914 (2008) Article DOI: 10.1021/jm8011036 BindingDB Entry DOI: 10.7270/Q2WS8T4C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| BMP-2-inducible protein kinase (Homo sapiens (Human)) | BDBM50511380 (CHEMBL4452360) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| BMP-2-inducible protein kinase (Homo sapiens (Human)) | BDBM50511383 (CHEMBL4531680) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50511378 (CHEMBL4470026) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50102194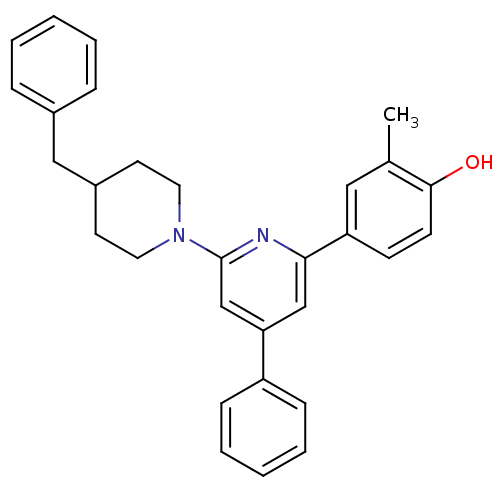 (4-(4-Benzyl-4'-phenyl-3,4,5,6-tetrahydro-2H-[1,2']...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Ability to displace [3H]-17-beta-estradiol from Estrogen receptor alpha by scintillation proximity assay. | Bioorg Med Chem Lett 11: 1939-42 (2001) BindingDB Entry DOI: 10.7270/Q2ZK5FZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50511399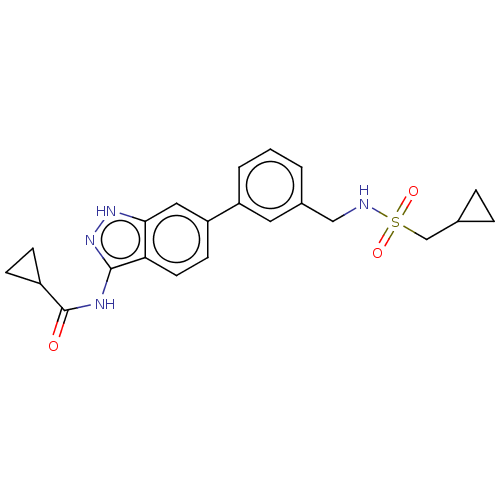 (CHEMBL4542463) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50511395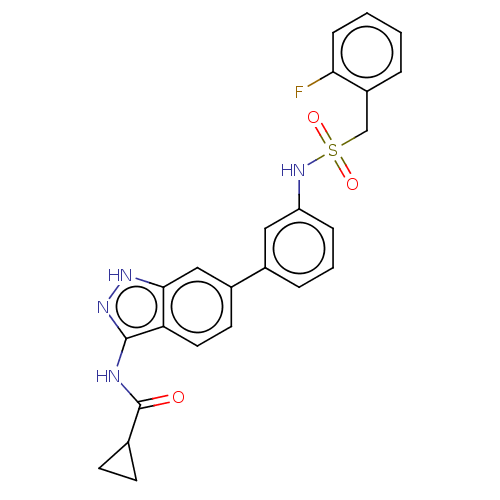 (CHEMBL4473140) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AP2-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50511375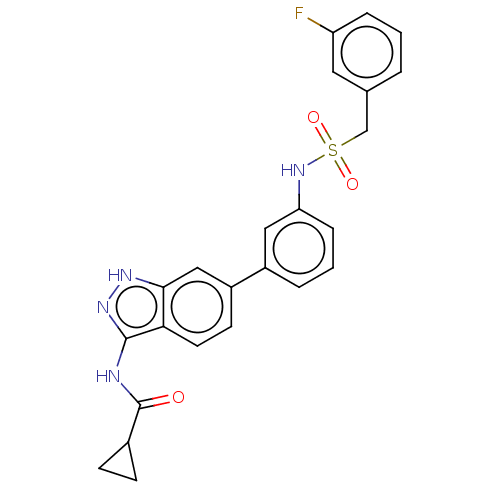 (CHEMBL4593649) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged AAK1 kinase domain (31 to 396 residues) (unknown origin)... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BMP-2-inducible protein kinase (Homo sapiens (Human)) | BDBM50511379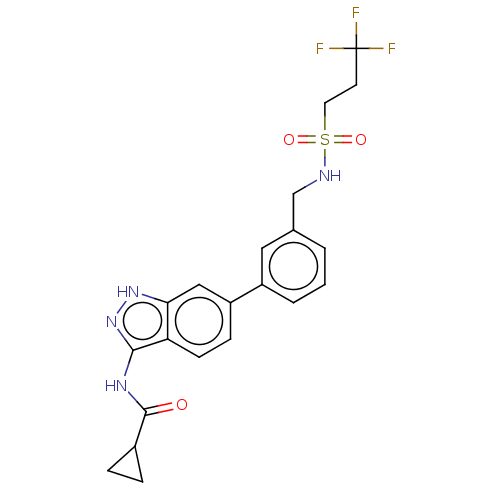 (CHEMBL4447403) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill (UNC-CH) Curated by ChEMBL | Assay Description Displacement of Alexafluor labelled kinase tracer236 from biotinylated C-terminal Avi-tagged BMP2K kinase domain (38 to 345 residues) (unknown origin... | ACS Med Chem Lett 11: 340-345 (2020) Article DOI: 10.1021/acsmedchemlett.9b00399 BindingDB Entry DOI: 10.7270/Q2ZC8669 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4527 total ) | Next | Last >> |