 Found 1190 hits with Last Name = 'rotili' and Initial = 'd'
Found 1190 hits with Last Name = 'rotili' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
NAD-dependent protein deacylase sirtuin-5, mitochondrial
(Homo sapiens (Human)) | BDBM50598638

(CHEMBL5199270)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H](CCCCNC(=S)NCCc1nnn[nH]1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC1CCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00687
BindingDB Entry DOI: 10.7270/Q2125XQ3 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM172038
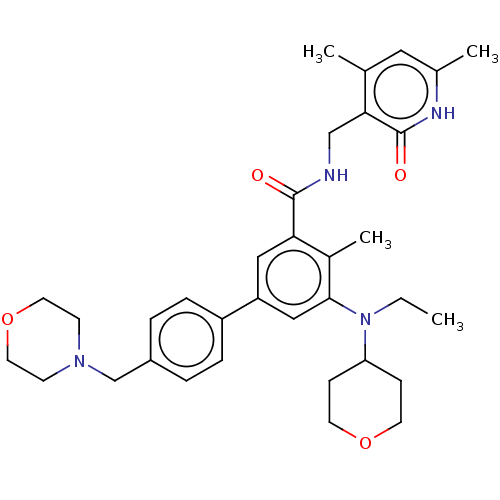
(US10155002, Compound 44 | US10647700, Compound EPZ...)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C34H44N4O4/c1-5-38(29-10-14-41-15-11-29)32-20-28(27-8-6-26(7-9-27)22-37-12-16-42-17-13-37)19-30(25(32)4)33(39)35-21-31-23(2)18-24(3)36-34(31)40/h6-9,18-20,29H,5,10-17,21-22H2,1-4H3,(H,35,39)(H,36,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477178

(CHEMBL239063)Show InChI InChI=1S/C17H19F2N3O2/c1-10(14-12(18)4-3-5-13(14)19)15-11(2)16(23)21-17(20-15)22-6-8-24-9-7-22/h3-5,10H,6-9H2,1-2H3,(H,20,21,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2325
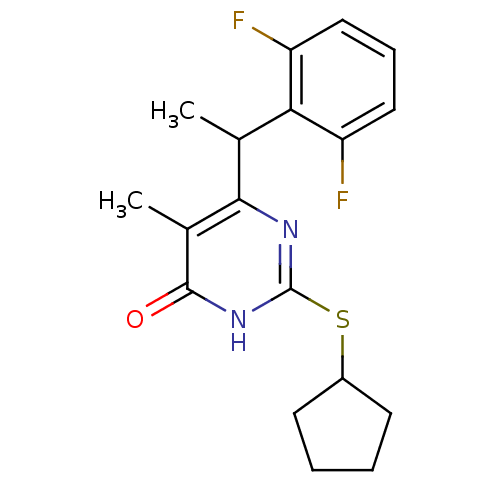
(2-(cyclopentylsulfanyl)-6-[1-(2,6-difluorophenyl)e...)Show InChI InChI=1S/C18H20F2N2OS/c1-10(15-13(19)8-5-9-14(15)20)16-11(2)17(23)22-18(21-16)24-12-6-3-4-7-12/h5,8-10,12H,3-4,6-7H2,1-2H3,(H,21,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 10: 661-665 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00023
BindingDB Entry DOI: 10.7270/Q2MK6H7P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NAD-dependent protein deacylase sirtuin-5, mitochondrial
(Homo sapiens (Human)) | BDBM50540057
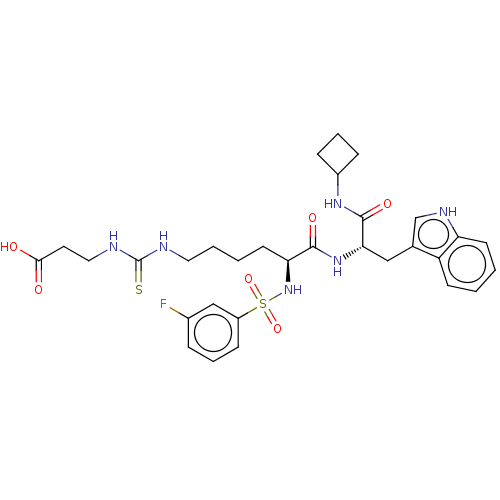
(CHEMBL4636862)Show SMILES OC(=O)CCNC(=S)NCCCC[C@H](NS(=O)(=O)c1cccc(F)c1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC1CCC1 |r| Show InChI InChI=1S/C31H39FN6O6S2/c32-21-7-5-10-23(18-21)46(43,44)38-26(13-3-4-15-33-31(45)34-16-14-28(39)40)29(41)37-27(30(42)36-22-8-6-9-22)17-20-19-35-25-12-2-1-11-24(20)25/h1-2,5,7,10-12,18-19,22,26-27,35,38H,3-4,6,8-9,13-17H2,(H,36,42)(H,37,41)(H,39,40)(H2,33,34,45)/t26-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00687
BindingDB Entry DOI: 10.7270/Q2125XQ3 |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacylase sirtuin-5, mitochondrial
(Homo sapiens (Human)) | BDBM50598639
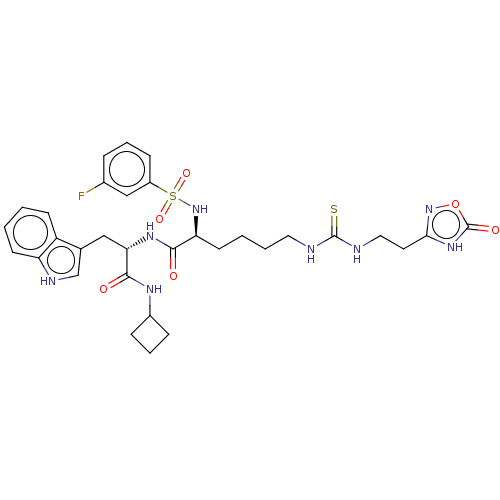
(CHEMBL5209031)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H](CCCCNC(=S)NCCc1noc(=O)[nH]1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NC1CCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00687
BindingDB Entry DOI: 10.7270/Q2125XQ3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50510060
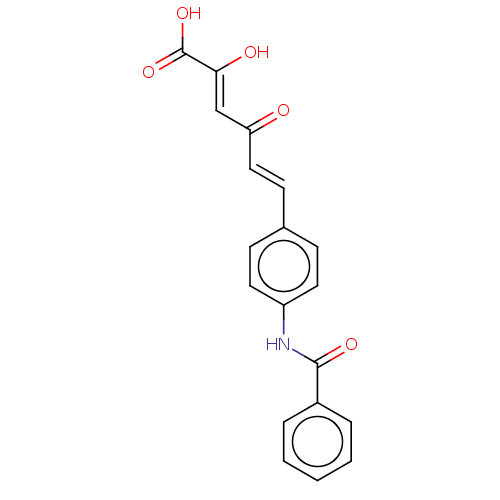
(CHEMBL4558305)Show SMILES OC(=O)C(\O)=C\C(=O)\C=C\c1ccc(NC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C19H15NO5/c21-16(12-17(22)19(24)25)11-8-13-6-9-15(10-7-13)20-18(23)14-4-2-1-3-5-14/h1-12,22H,(H,20,23)(H,24,25)/b11-8+,17-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 10: 661-665 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00023
BindingDB Entry DOI: 10.7270/Q2MK6H7P |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477201

(CHEMBL394990)Show InChI InChI=1S/C16H19F2N3O/c1-4-8-21(3)16-19-14(10(2)15(22)20-16)9-11-12(17)6-5-7-13(11)18/h5-7H,4,8-9H2,1-3H3,(H,19,20,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V106A mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50510063
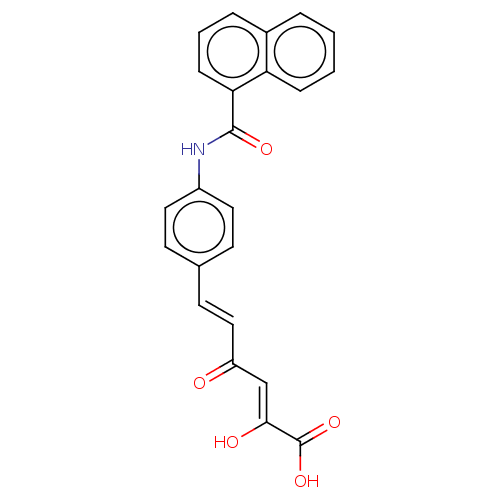
(CHEMBL4568798)Show SMILES OC(=O)C(\O)=C\C(=O)\C=C\c1ccc(NC(=O)c2cccc3ccccc23)cc1 Show InChI InChI=1S/C23H17NO5/c25-18(14-21(26)23(28)29)13-10-15-8-11-17(12-9-15)24-22(27)20-7-3-5-16-4-1-2-6-19(16)20/h1-14,26H,(H,24,27)(H,28,29)/b13-10+,21-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 10: 661-665 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00023
BindingDB Entry DOI: 10.7270/Q2MK6H7P |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50510058
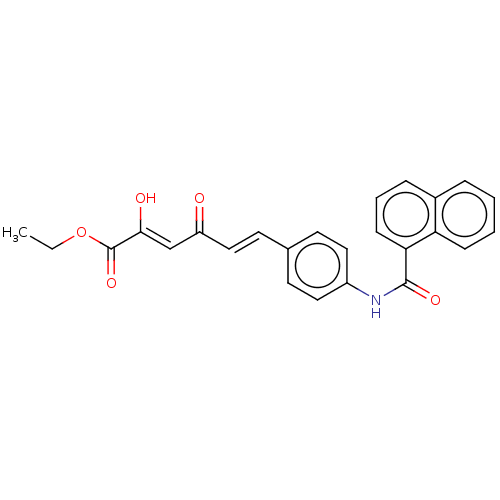
(CHEMBL4439915)Show SMILES CCOC(=O)C(\O)=C\C(=O)\C=C\c1ccc(NC(=O)c2cccc3ccccc23)cc1 Show InChI InChI=1S/C25H21NO5/c1-2-31-25(30)23(28)16-20(27)15-12-17-10-13-19(14-11-17)26-24(29)22-9-5-7-18-6-3-4-8-21(18)22/h3-16,28H,2H2,1H3,(H,26,29)/b15-12+,23-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 10: 661-665 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00023
BindingDB Entry DOI: 10.7270/Q2MK6H7P |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477199
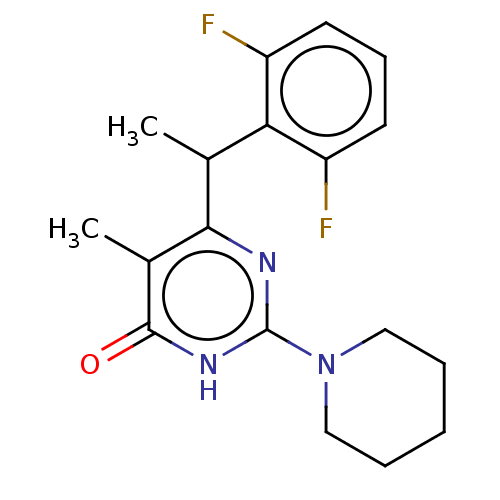
(CHEMBL239342)Show InChI InChI=1S/C18H21F2N3O/c1-11(15-13(19)7-6-8-14(15)20)16-12(2)17(24)22-18(21-16)23-9-4-3-5-10-23/h6-8,11H,3-5,9-10H2,1-2H3,(H,21,22,24) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V179D mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477179

(CHEMBL399016)Show SMILES Cc1c(Cc2c(F)cccc2F)nc([nH]c1=O)N1CCN(CC1)c1ccccn1 Show InChI InChI=1S/C21H21F2N5O/c1-14-18(13-15-16(22)5-4-6-17(15)23)25-21(26-20(14)29)28-11-9-27(10-12-28)19-7-2-3-8-24-19/h2-8H,9-13H2,1H3,(H,25,26,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V106A mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477179

(CHEMBL399016)Show SMILES Cc1c(Cc2c(F)cccc2F)nc([nH]c1=O)N1CCN(CC1)c1ccccn1 Show InChI InChI=1S/C21H21F2N5O/c1-14-18(13-15-16(22)5-4-6-17(15)23)25-21(26-20(14)29)28-11-9-27(10-12-28)19-7-2-3-8-24-19/h2-8H,9-13H2,1H3,(H,25,26,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V179D mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50510062
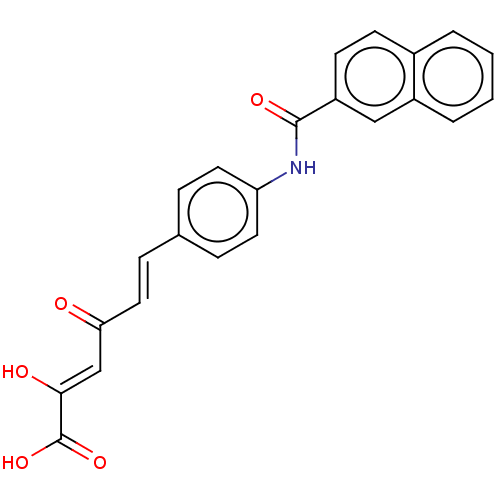
(CHEMBL4575531)Show SMILES OC(=O)C(\O)=C\C(=O)\C=C\c1ccc(NC(=O)c2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C23H17NO5/c25-20(14-21(26)23(28)29)12-7-15-5-10-19(11-6-15)24-22(27)18-9-8-16-3-1-2-4-17(16)13-18/h1-14,26H,(H,24,27)(H,28,29)/b12-7+,21-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 10: 661-665 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00023
BindingDB Entry DOI: 10.7270/Q2MK6H7P |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 10: 661-665 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00023
BindingDB Entry DOI: 10.7270/Q2MK6H7P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50510060

(CHEMBL4558305)Show SMILES OC(=O)C(\O)=C\C(=O)\C=C\c1ccc(NC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C19H15NO5/c21-16(12-17(22)19(24)25)11-8-13-6-9-15(10-7-13)20-18(23)14-4-2-1-3-5-14/h1-12,22H,(H,20,23)(H,24,25)/b11-8+,17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 10: 661-665 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00023
BindingDB Entry DOI: 10.7270/Q2MK6H7P |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50510061
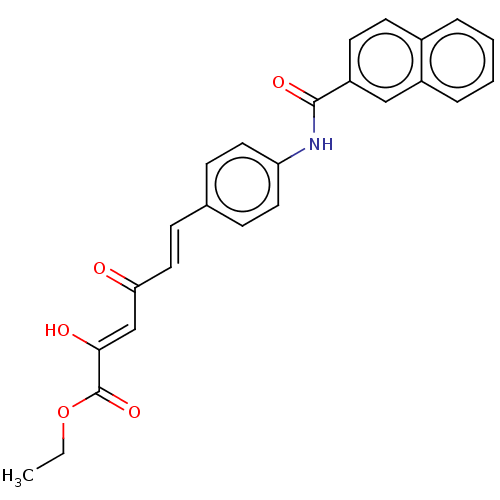
(CHEMBL4578890)Show SMILES CCOC(=O)C(\O)=C\C(=O)\C=C\c1ccc(NC(=O)c2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C25H21NO5/c1-2-31-25(30)23(28)16-22(27)14-9-17-7-12-21(13-8-17)26-24(29)20-11-10-18-5-3-4-6-19(18)15-20/h3-16,28H,2H2,1H3,(H,26,29)/b14-9+,23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 10: 661-665 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00023
BindingDB Entry DOI: 10.7270/Q2MK6H7P |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50510063

(CHEMBL4568798)Show SMILES OC(=O)C(\O)=C\C(=O)\C=C\c1ccc(NC(=O)c2cccc3ccccc23)cc1 Show InChI InChI=1S/C23H17NO5/c25-18(14-21(26)23(28)29)13-10-15-8-11-17(12-9-15)24-22(27)20-7-3-5-16-4-1-2-6-19(16)20/h1-14,26H,(H,24,27)(H,28,29)/b13-10+,21-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 10: 661-665 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00023
BindingDB Entry DOI: 10.7270/Q2MK6H7P |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT8
(Homo sapiens (Human)) | BDBM43339

(4-amino-1-naphthalenol;hydrochloride | 4-amino-1-n...)Show InChI InChI=1S/C10H9NO/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6,12H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged KAT8 catalytic domain (125 to 458 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) assessed as... |
Eur J Med Chem 136: 480-486 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.015
BindingDB Entry DOI: 10.7270/Q20Z75S9 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477200

(CHEMBL396816)Show SMILES CCCN(C)c1nc(C(C)c2c(F)cccc2F)c(C)c(=O)[nH]1 Show InChI InChI=1S/C17H21F2N3O/c1-5-9-22(4)17-20-15(11(3)16(23)21-17)10(2)14-12(18)7-6-8-13(14)19/h6-8,10H,5,9H2,1-4H3,(H,20,21,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V106A mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477198

(CHEMBL239064)Show InChI InChI=1S/C17H19F2N3OS/c1-10(14-12(18)4-3-5-13(14)19)15-11(2)16(23)21-17(20-15)22-6-8-24-9-7-22/h3-5,10H,6-9H2,1-2H3,(H,20,21,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V179D mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477188

(CHEMBL239337)Show InChI InChI=1S/C16H19F2N3O/c1-5-21(4)16-19-14(10(3)15(22)20-16)9(2)13-11(17)7-6-8-12(13)18/h6-9H,5H2,1-4H3,(H,19,20,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V179D mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477199

(CHEMBL239342)Show InChI InChI=1S/C18H21F2N3O/c1-11(15-13(19)7-6-8-14(15)20)16-12(2)17(24)22-18(21-16)23-9-4-3-5-10-23/h6-8,11H,3-5,9-10H2,1-2H3,(H,21,22,24) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V106A mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477188

(CHEMBL239337)Show InChI InChI=1S/C16H19F2N3O/c1-5-21(4)16-19-14(10(3)15(22)20-16)9(2)13-11(17)7-6-8-12(13)18/h6-9H,5H2,1-4H3,(H,19,20,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V106A mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacylase sirtuin-5, mitochondrial
(Homo sapiens (Human)) | BDBM50598635

(CHEMBL5172115)Show SMILES CC(C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCCNC(=S)CCCC(O)=O)NC(=O)OCc1ccccc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00687
BindingDB Entry DOI: 10.7270/Q2125XQ3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 10: 661-665 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00023
BindingDB Entry DOI: 10.7270/Q2MK6H7P |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477198

(CHEMBL239064)Show InChI InChI=1S/C17H19F2N3OS/c1-10(14-12(18)4-3-5-13(14)19)15-11(2)16(23)21-17(20-15)22-6-8-24-9-7-22/h3-5,10H,6-9H2,1-2H3,(H,20,21,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V106A mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477200

(CHEMBL396816)Show SMILES CCCN(C)c1nc(C(C)c2c(F)cccc2F)c(C)c(=O)[nH]1 Show InChI InChI=1S/C17H21F2N3O/c1-5-9-22(4)17-20-15(11(3)16(23)21-17)10(2)14-12(18)7-6-8-13(14)19/h6-8,10H,5,9H2,1-4H3,(H,20,21,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477185

(CHEMBL239338)Show SMILES CC(C)N(C)c1nc(C(C)c2c(F)cccc2F)c(C)c(=O)[nH]1 Show InChI InChI=1S/C17H21F2N3O/c1-9(2)22(5)17-20-15(11(4)16(23)21-17)10(3)14-12(18)7-6-8-13(14)19/h6-10H,1-5H3,(H,20,21,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V106A mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477196
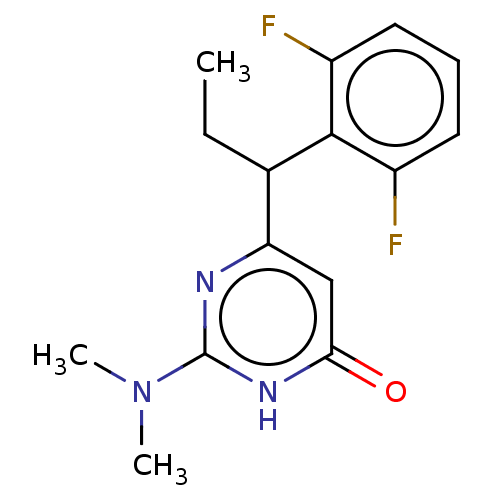
(CHEMBL393696)Show InChI InChI=1S/C15H17F2N3O/c1-4-9(14-10(16)6-5-7-11(14)17)12-8-13(21)19-15(18-12)20(2)3/h5-9H,4H2,1-3H3,(H,18,19,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V179D mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM2325

(2-(cyclopentylsulfanyl)-6-[1-(2,6-difluorophenyl)e...)Show InChI InChI=1S/C18H20F2N2OS/c1-10(15-13(19)8-5-9-14(15)20)16-11(2)17(23)22-18(21-16)24-12-6-3-4-7-12/h5,8-10,12H,3-4,6-7H2,1-2H3,(H,21,22,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase K103N mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477186

(CHEMBL238907)Show InChI InChI=1S/C16H19F2N3O/c1-5-10-14(19-16(21(3)4)20-15(10)22)9(2)13-11(17)7-6-8-12(13)18/h6-9H,5H2,1-4H3,(H,19,20,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V179D mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
REST corepressor 1
(Homo sapiens (Human)) | BDBM50262048
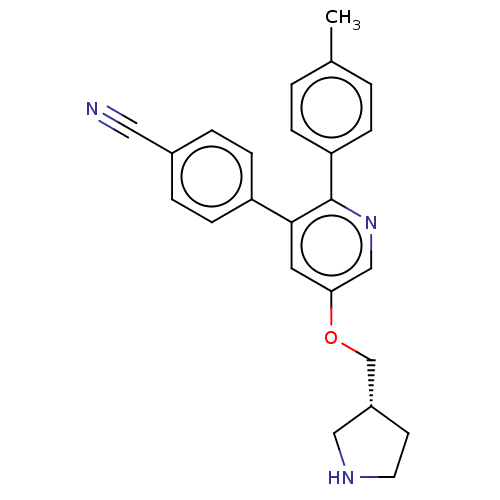
(CHEMBL3134377)Show SMILES Cc1ccc(cc1)-c1ncc(OC[C@@H]2CCNC2)cc1-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C24H23N3O/c1-17-2-6-21(7-3-17)24-23(20-8-4-18(13-25)5-9-20)12-22(15-27-24)28-16-19-10-11-26-14-19/h2-9,12,15,19,26H,10-11,14,16H2,1H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114410
BindingDB Entry DOI: 10.7270/Q2C53QW7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50510059
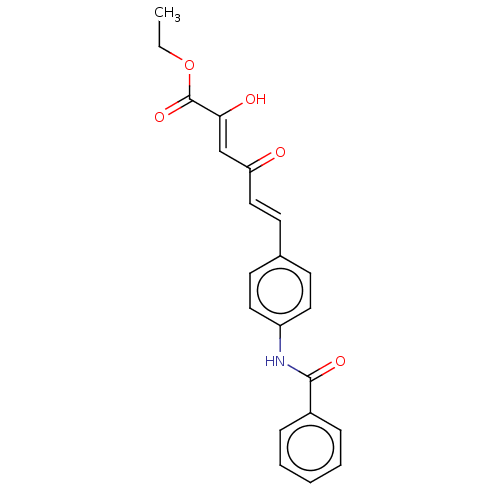
(CHEMBL4578633)Show SMILES CCOC(=O)C(\O)=C\C(=O)\C=C\c1ccc(NC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C21H19NO5/c1-2-27-21(26)19(24)14-18(23)13-10-15-8-11-17(12-9-15)22-20(25)16-6-4-3-5-7-16/h3-14,24H,2H2,1H3,(H,22,25)/b13-10+,19-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 10: 661-665 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00023
BindingDB Entry DOI: 10.7270/Q2MK6H7P |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacylase sirtuin-5, mitochondrial
(Homo sapiens (Human)) | BDBM50598634
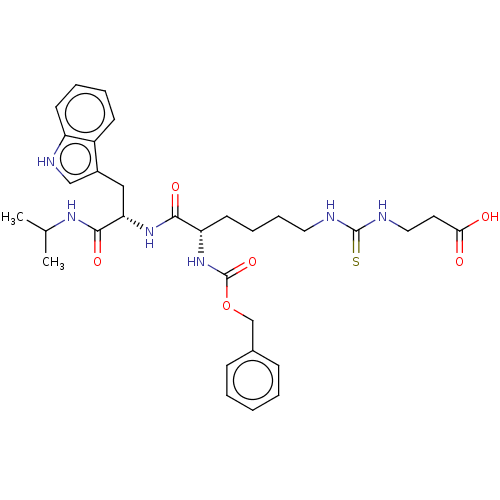
(CHEMBL5188575)Show SMILES CC(C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCCNC(=S)NCCC(O)=O)NC(=O)OCc1ccccc1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00687
BindingDB Entry DOI: 10.7270/Q2125XQ3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50510058

(CHEMBL4439915)Show SMILES CCOC(=O)C(\O)=C\C(=O)\C=C\c1ccc(NC(=O)c2cccc3ccccc23)cc1 Show InChI InChI=1S/C25H21NO5/c1-2-31-25(30)23(28)16-20(27)15-12-17-10-13-19(14-11-17)26-24(29)22-9-5-7-18-6-3-4-8-21(18)22/h3-16,28H,2H2,1H3,(H,26,29)/b15-12+,23-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 incubated for 15 mins by stopped flow CO2 hydrase assay |
ACS Med Chem Lett 10: 661-665 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00023
BindingDB Entry DOI: 10.7270/Q2MK6H7P |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477189
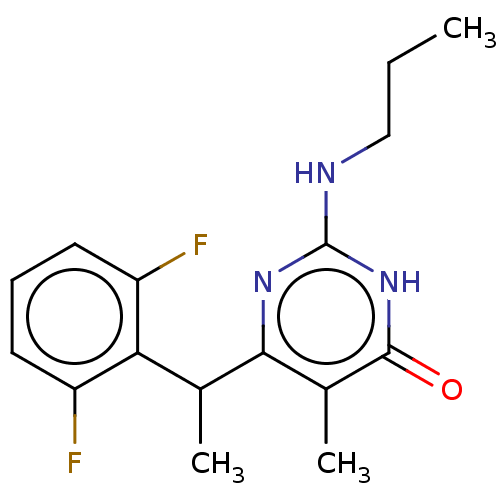
(CHEMBL239281)Show InChI InChI=1S/C16H19F2N3O/c1-4-8-19-16-20-14(10(3)15(22)21-16)9(2)13-11(17)6-5-7-12(13)18/h5-7,9H,4,8H2,1-3H3,(H2,19,20,21,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477188

(CHEMBL239337)Show InChI InChI=1S/C16H19F2N3O/c1-5-21(4)16-19-14(10(3)15(22)20-16)9(2)13-11(17)7-6-8-12(13)18/h6-9H,5H2,1-4H3,(H,19,20,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477185

(CHEMBL239338)Show SMILES CC(C)N(C)c1nc(C(C)c2c(F)cccc2F)c(C)c(=O)[nH]1 Show InChI InChI=1S/C17H21F2N3O/c1-9(2)22(5)17-20-15(11(4)16(23)21-17)10(3)14-12(18)7-6-8-13(14)19/h6-10H,1-5H3,(H,20,21,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477189

(CHEMBL239281)Show InChI InChI=1S/C16H19F2N3O/c1-4-8-19-16-20-14(10(3)15(22)21-16)9(2)13-11(17)6-5-7-12(13)18/h5-7,9H,4,8H2,1-3H3,(H2,19,20,21,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V106A mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477179

(CHEMBL399016)Show SMILES Cc1c(Cc2c(F)cccc2F)nc([nH]c1=O)N1CCN(CC1)c1ccccn1 Show InChI InChI=1S/C21H21F2N5O/c1-14-18(13-15-16(22)5-4-6-17(15)23)25-21(26-20(14)29)28-11-9-27(10-12-28)19-7-2-3-8-24-19/h2-8H,9-13H2,1H3,(H,25,26,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477186

(CHEMBL238907)Show InChI InChI=1S/C16H19F2N3O/c1-5-10-14(19-16(21(3)4)20-15(10)22)9(2)13-11(17)7-6-8-12(13)18/h6-9H,5H2,1-4H3,(H,19,20,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477180

(CHEMBL238701)Show InChI InChI=1S/C16H17F2N3O2/c1-10-14(9-11-12(17)3-2-4-13(11)18)19-16(20-15(10)22)21-5-7-23-8-6-21/h2-4H,5-9H2,1H3,(H,19,20,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477198

(CHEMBL239064)Show InChI InChI=1S/C17H19F2N3OS/c1-10(14-12(18)4-3-5-13(14)19)15-11(2)16(23)21-17(20-15)22-6-8-24-9-7-22/h3-5,10H,6-9H2,1-2H3,(H,20,21,23) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477186

(CHEMBL238907)Show InChI InChI=1S/C16H19F2N3O/c1-5-10-14(19-16(21(3)4)20-15(10)22)9(2)13-11(17)7-6-8-12(13)18/h6-9H,5H2,1-4H3,(H,19,20,22) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase V106A mutant |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477199

(CHEMBL239342)Show InChI InChI=1S/C18H21F2N3O/c1-11(15-13(19)7-6-8-14(15)20)16-12(2)17(24)22-18(21-16)23-9-4-3-5-10-23/h6-8,11H,3-5,9-10H2,1-2H3,(H,21,22,24) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50477197

(CHEMBL391164)Show InChI InChI=1S/C13H13F2N3O/c1-18(2)13-16-8(7-12(19)17-13)6-9-10(14)4-3-5-11(9)15/h3-5,7H,6H2,1-2H3,(H,16,17,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 3B reverse transcriptase |
J Med Chem 50: 5412-24 (2007)
Article DOI: 10.1021/jm070811e
BindingDB Entry DOI: 10.7270/Q2H99803 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data