

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (Rattus norvegicus) | BDBM22417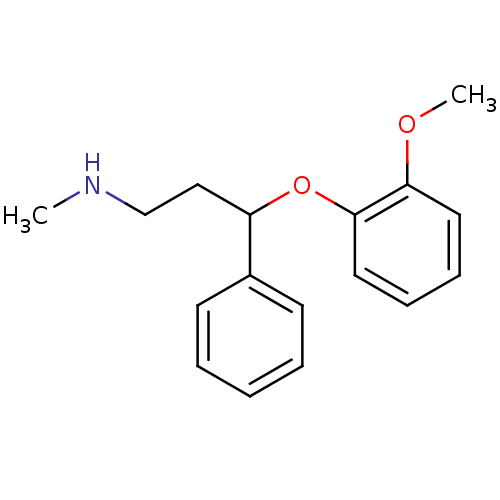 (3-(2-methoxyphenoxy)-N-methyl-3-phenylpropan-1-ami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00072a BindingDB Entry DOI: 10.7270/Q2N301ZH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50021246 (3-(3,3-dimethyl-1-phenyl-1,3-dihydroisobenzofuran-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00072a BindingDB Entry DOI: 10.7270/Q2N301ZH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50175155 (2-(3-(bis(4-fluorophenyl)methoxy)-8-aza-bicyclo[3....) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50138641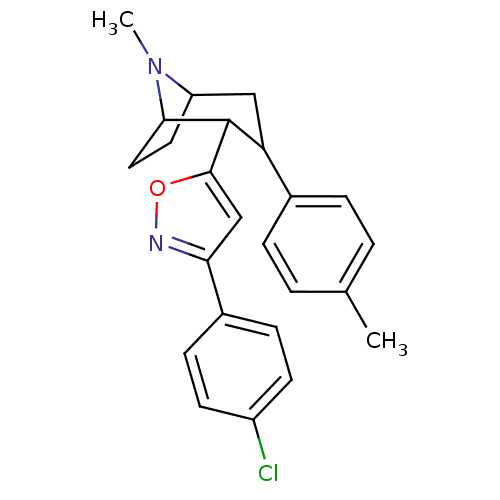 (2-[3-(4-Chloro-phenyl)-isoxazol-5-yl]-8-methyl-3-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50138641 (2-[3-(4-Chloro-phenyl)-isoxazol-5-yl]-8-methyl-3-p...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]DA uptake at wild type human DAT expressed in African green monkey COS7 cells after 5 mins by beta scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50247032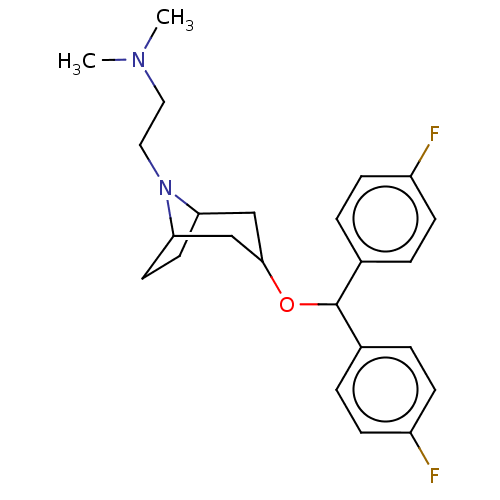 (CHEMBL4062435) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]WIN5428 uptake at wild type human DAT expressed in African green monkey COS7 cells after 90 mins | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50138650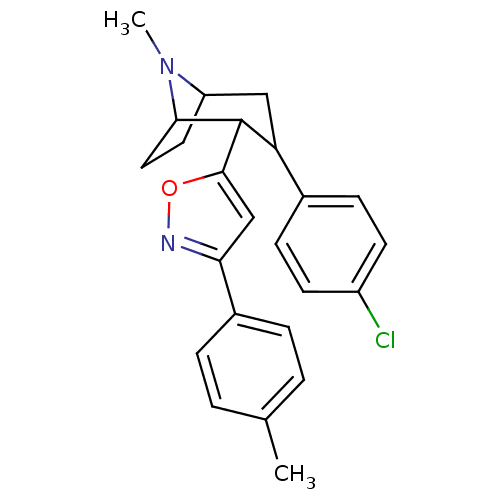 (3-(4-Chloro-phenyl)-8-methyl-2-(3-p-tolyl-isoxazol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247040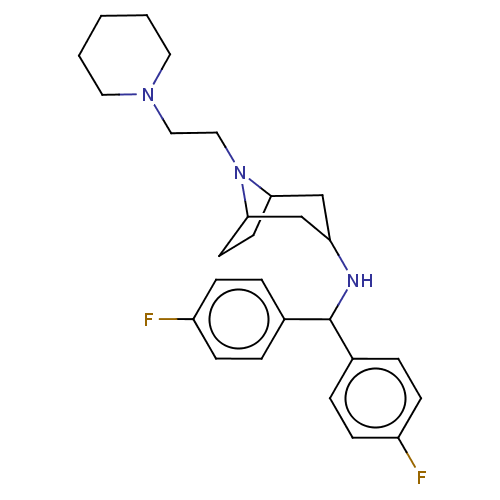 (CHEMBL4082578) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247026 (CHEMBL4077089) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247032 (CHEMBL4062435) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247030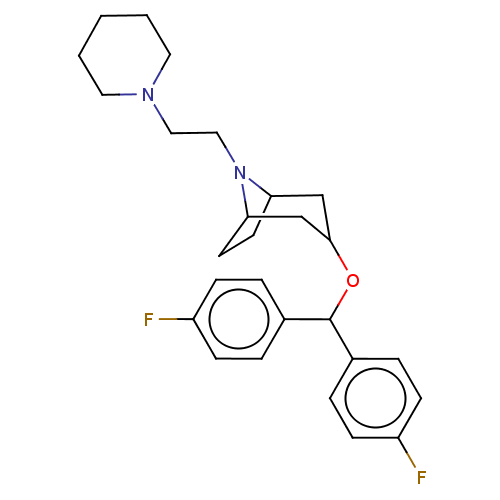 (CHEMBL4100591) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50086042 ((R)-1-{3-[Bis-(4-fluoro-phenyl)-methoxy]-8-aza-bic...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247043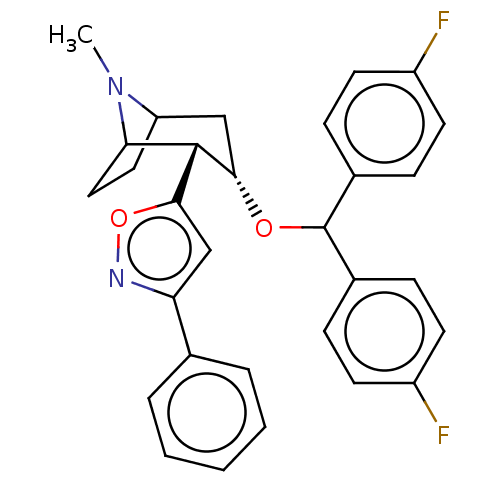 (CHEMBL4074774) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247031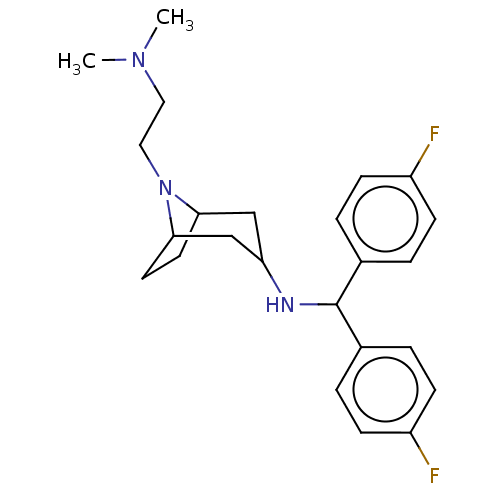 (CHEMBL4090416) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247023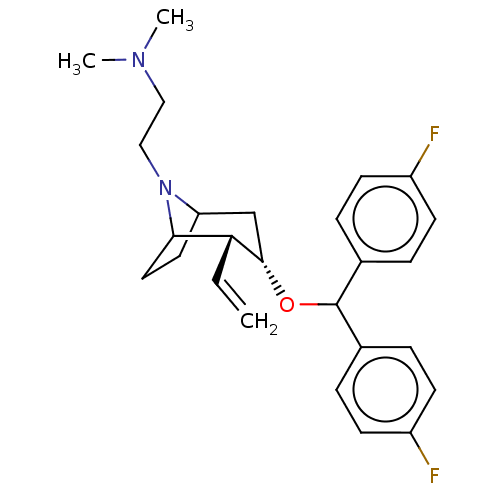 (CHEMBL4070531) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247043 (CHEMBL4074774) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50138650 (3-(4-Chloro-phenyl)-8-methyl-2-(3-p-tolyl-isoxazol...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]DA uptake at wild type human DAT expressed in African green monkey COS7 cells after 5 mins by beta scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50247031 (CHEMBL4090416) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]WIN5428 uptake at wild type human DAT expressed in African green monkey COS7 cells after 90 mins | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50177767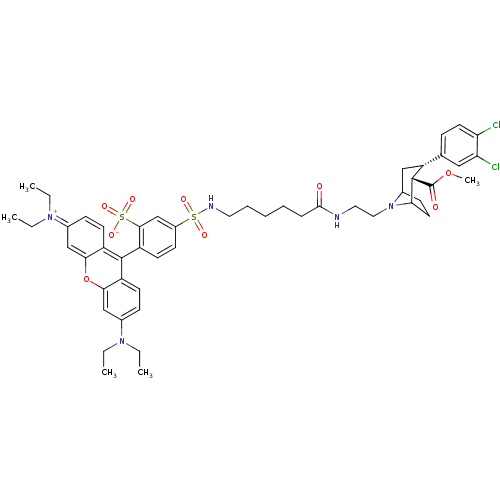 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00072a BindingDB Entry DOI: 10.7270/Q2N301ZH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247024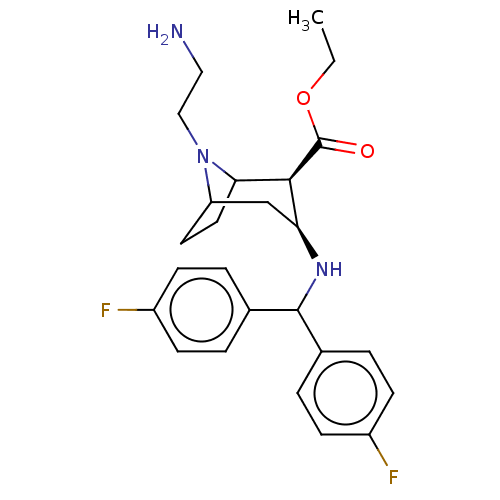 (CHEMBL4097211) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50177767 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00072a BindingDB Entry DOI: 10.7270/Q2N301ZH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247027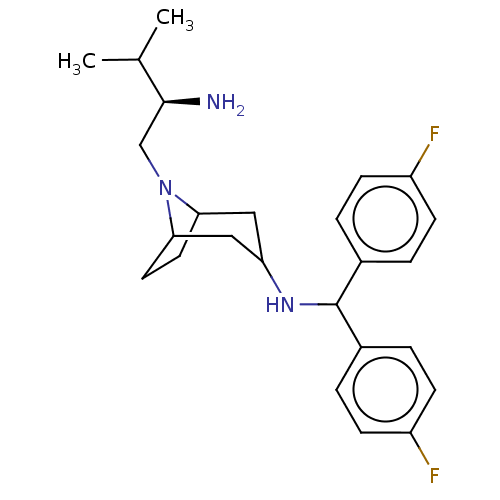 (CHEMBL4092855) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50175157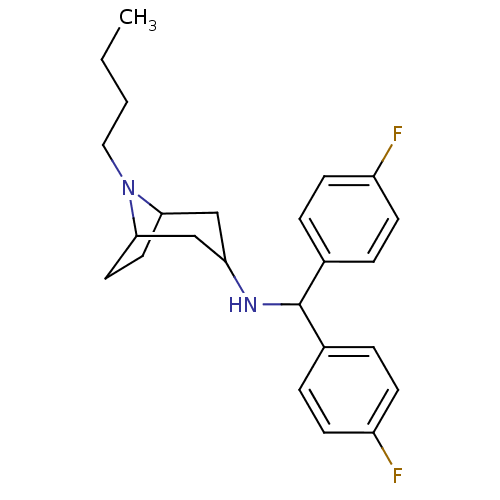 (CHEMBL371932 | N-(bis(4-fluorophenyl)methyl)-8-but...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]WIN5428 uptake at wild type human DAT expressed in African green monkey COS7 cells after 90 mins | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50061881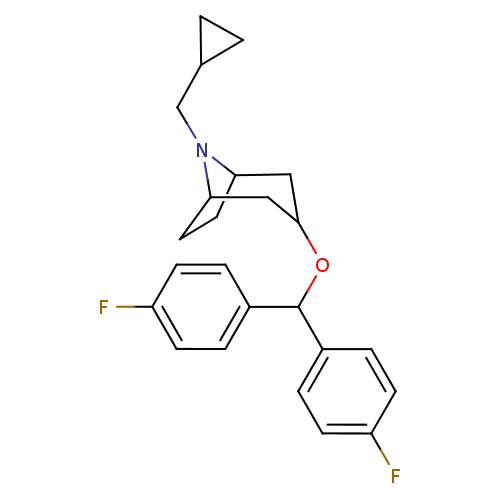 (3-[Bis-(4-fluoro-phenyl)-methoxy]-8-cyclopropylmet...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247039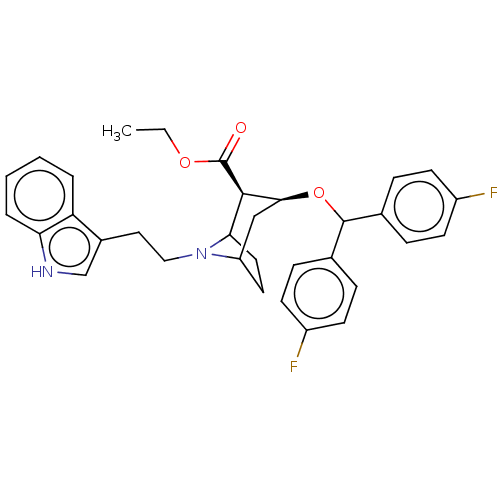 (CHEMBL4085107) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50138641 (2-[3-(4-Chloro-phenyl)-isoxazol-5-yl]-8-methyl-3-p...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]WIN5428 uptake at wild type human DAT expressed in African green monkey COS7 cells after 90 mins | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50175159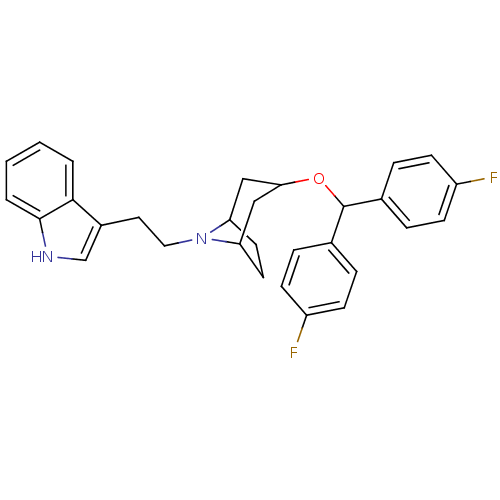 (3-(2-{3-[Bis-(4-fluoro-phenyl)-methoxy]-8-aza-bicy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50138650 (3-(4-Chloro-phenyl)-8-methyl-2-(3-p-tolyl-isoxazol...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]WIN5428 uptake at wild type human DAT expressed in African green monkey COS7 cells after 90 mins | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50247031 (CHEMBL4090416) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]DA uptake at wild type human DAT expressed in African green monkey COS7 cells after 5 mins by beta scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50597616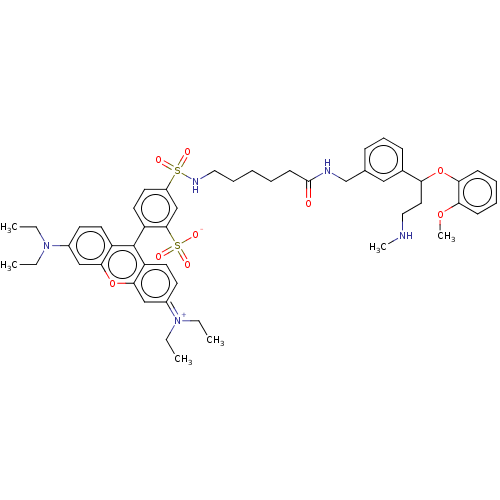 (CHEMBL5188712) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00072a BindingDB Entry DOI: 10.7270/Q2N301ZH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50247032 (CHEMBL4062435) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]DA uptake at wild type human DAT expressed in African green monkey COS7 cells after 5 mins by beta scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247025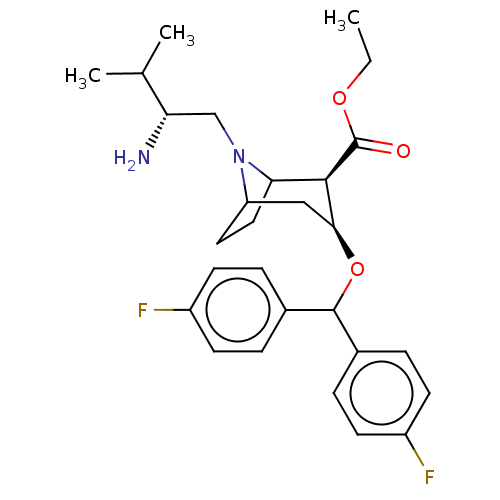 (CHEMBL4063478) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50175157 (CHEMBL371932 | N-(bis(4-fluorophenyl)methyl)-8-but...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]DA uptake at wild type human DAT expressed in African green monkey COS7 cells after 5 mins by beta scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247068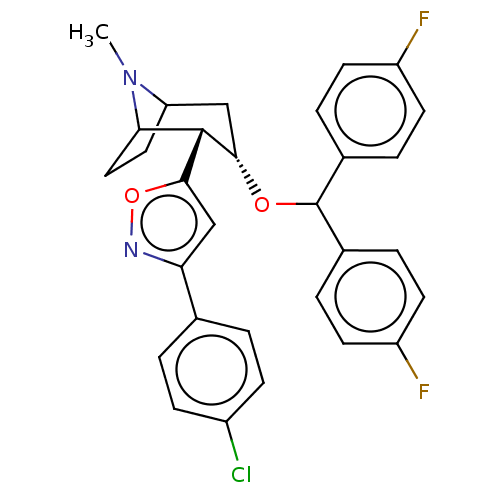 (CHEMBL4085035) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247041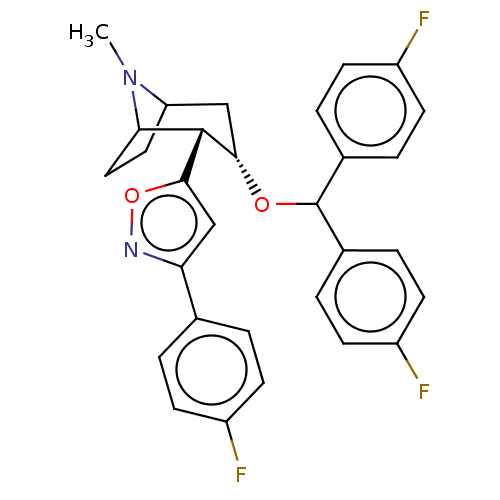 (CHEMBL4063396) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50177767 (2-(3-sec-Butylimino-6-diethylamino-3H-xanthen-9-yl...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00072a BindingDB Entry DOI: 10.7270/Q2N301ZH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50086042 ((R)-1-{3-[Bis-(4-fluoro-phenyl)-methoxy]-8-aza-bic...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]WIN5428 uptake at wild type human DAT expressed in African green monkey COS7 cells after 90 mins | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50247042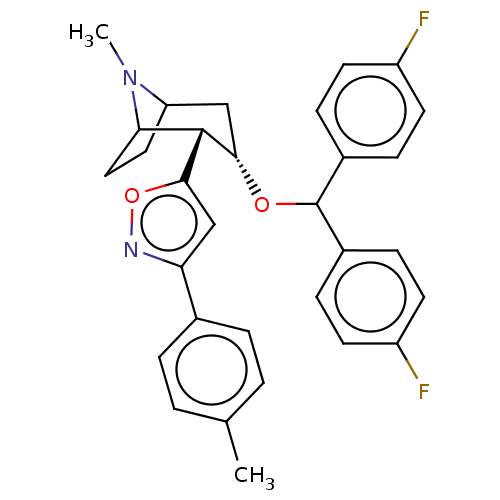 (CHEMBL4101457) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from Sprague-Dawley rat brain DAT after 120 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50086042 ((R)-1-{3-[Bis-(4-fluoro-phenyl)-methoxy]-8-aza-bic...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]DA uptake at wild type human DAT expressed in African green monkey COS7 cells after 5 mins by beta scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50247039 (CHEMBL4085107) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]WIN5428 uptake at wild type human DAT expressed in African green monkey COS7 cells after 90 mins | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50061885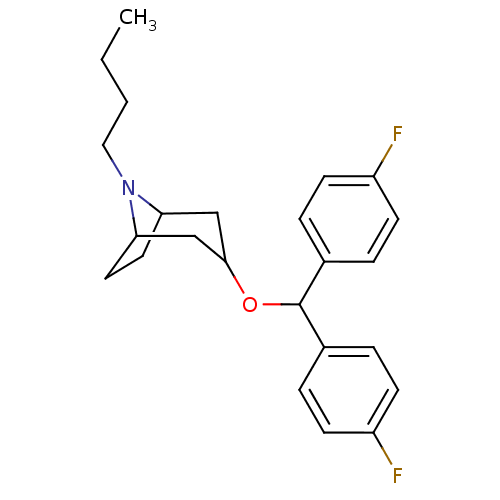 (3-[Bis-(4-fluoro-phenyl)-methoxy]-8-butyl-8-aza-bi...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]DA uptake at wild type human DAT expressed in African green monkey COS7 cells after 5 mins by beta scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50247025 (CHEMBL4063478) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]WIN5428 uptake at wild type human DAT expressed in African green monkey COS7 cells after 90 mins | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50175159 (3-(2-{3-[Bis-(4-fluoro-phenyl)-methoxy]-8-aza-bicy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 253 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]WIN5428 uptake at wild type human DAT expressed in African green monkey COS7 cells after 90 mins | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50175159 (3-(2-{3-[Bis-(4-fluoro-phenyl)-methoxy]-8-aza-bicy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat brain SERT after 60 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50247039 (CHEMBL4085107) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 746 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat brain SERT after 60 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50247068 (CHEMBL4085035) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 756 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat brain SERT after 60 mins by liquid scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50597616 (CHEMBL5188712) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 785 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00072a BindingDB Entry DOI: 10.7270/Q2N301ZH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50021246 (3-(3,3-dimethyl-1-phenyl-1,3-dihydroisobenzofuran-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 795 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00072a BindingDB Entry DOI: 10.7270/Q2N301ZH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50247039 (CHEMBL4085107) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 944 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Inhibition of [3H]DA uptake at wild type human DAT expressed in African green monkey COS7 cells after 5 mins by beta scintillation counting method | J Med Chem 60: 10172-10187 (2017) Article DOI: 10.1021/acs.jmedchem.7b01454 BindingDB Entry DOI: 10.7270/Q2NG4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50597618 (CHEMBL5176356) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 964 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00072a BindingDB Entry DOI: 10.7270/Q2N301ZH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 86 total ) | Next | Last >> |