

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-Hydroxyacid oxidase 1 (Homo sapiens (Human)) | BDBM50463046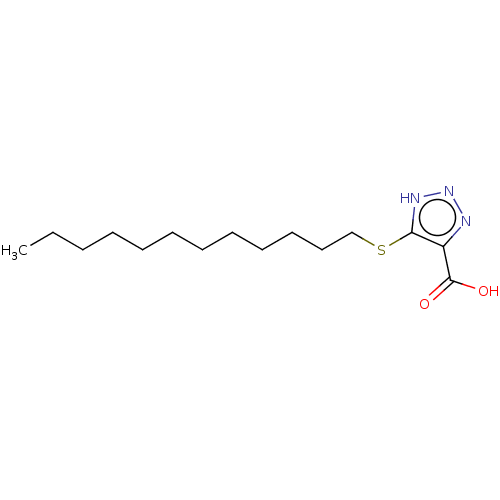 (CHEMBL1229989) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human glycolate oxidase expressed in Escherichia coli using glycolate as substrate by DCIP dye based spectrophotometry analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 2-Hydroxyacid oxidase 1 (Mus musculus) | BDBM50463047 (CHEMBL1794748) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non competitive inhibition of mouse glycolate oxidase in hyperoxaluric-Agxt knockdown mouse primary hepatocyte assessed as reduction in oxalate produ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50572932 (CHEMBL4850573) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50572931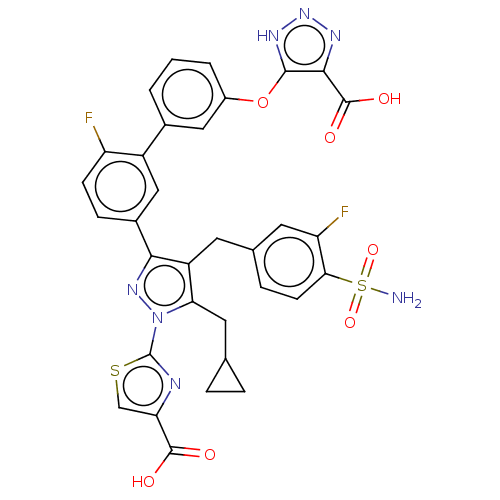 (CHEMBL4855986) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Mus musculus (Mouse)) | BDBM50250656 (CHEMBL4081890 | US11247971, Cmpd ID 276) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| L-lactate dehydrogenase A chain (Mus musculus (Mouse)) | BDBM50572931 (CHEMBL4855986) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Mus musculus (Mouse)) | BDBM50572933 (CHEMBL4861379) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Mus musculus (Mouse)) | BDBM50572930 (CHEMBL4873879) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50572933 (CHEMBL4861379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50572934 (CHEMBL4847068) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50572930 (CHEMBL4873879) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Mus musculus (Mouse)) | BDBM50572934 (CHEMBL4847068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Mus musculus (Mouse)) | BDBM50572932 (CHEMBL4850573) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50572929 (CHEMBL4864519) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50258579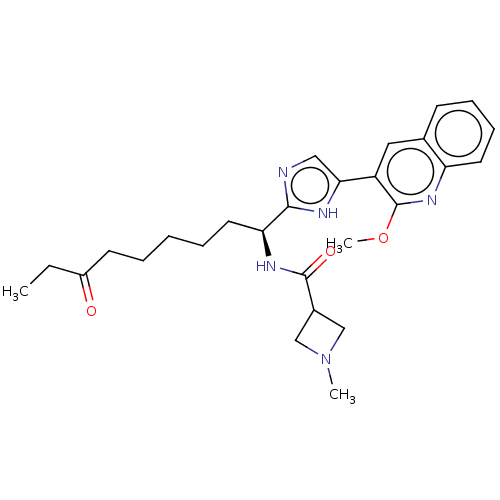 ((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Mus musculus (Mouse)) | BDBM50572929 (CHEMBL4864519) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse LDHA expressed in BL21 gold (DE3) using sodium pyruvate as substrate preincubated for 10 mins followed by sodium pyru... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-Hydroxyacid oxidase 1 (Homo sapiens (Human)) | BDBM50572931 (CHEMBL4855986) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GOX expressed in Escherichia coli C41 (DE3) using glycolate as substrate preincubated for 10 mins followed by substra... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50362592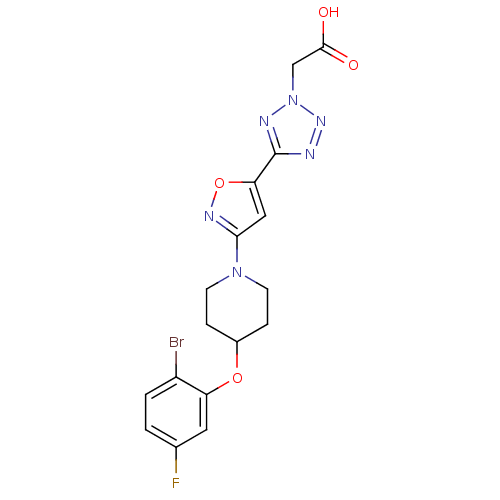 (CHEMBL1938870) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human SCD-1 | J Med Chem 54: 5082-96 (2011) Article DOI: 10.1021/jm200319u BindingDB Entry DOI: 10.7270/Q2348MGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50569630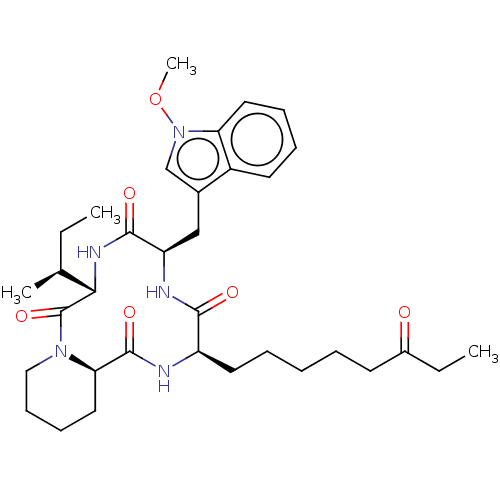 (CHEMBL4873847) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-Hydroxyacid oxidase 1 (Homo sapiens (Human)) | BDBM50572933 (CHEMBL4861379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GOX expressed in Escherichia coli C41 (DE3) using glycolate as substrate preincubated for 10 mins followed by substra... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-Hydroxyacid oxidase 1 (Homo sapiens (Human)) | BDBM50572932 (CHEMBL4850573) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GOX expressed in Escherichia coli C41 (DE3) using glycolate as substrate preincubated for 10 mins followed by substra... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50569630 (CHEMBL4873847) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50305768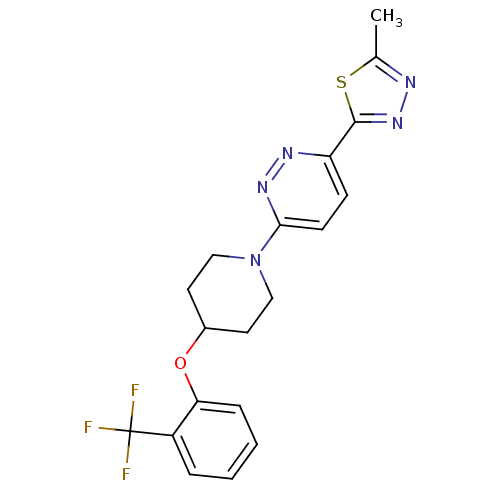 (2-methyl-5-(6-(4-(2-(trifluoromethyl)phenoxy)piper...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in Sprague-Dawley rat microsome assessed as reduction in [I-14C] stearoyl CoA desaturation by scintillation counting | J Med Chem 54: 5082-96 (2011) Article DOI: 10.1021/jm200319u BindingDB Entry DOI: 10.7270/Q2348MGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50569643 (CHEMBL4867665) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50258579 ((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50103568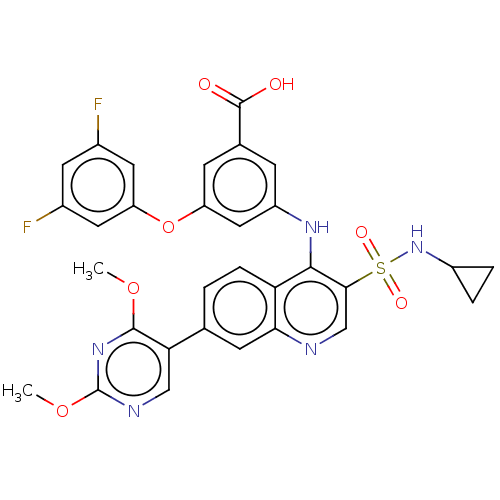 (CHEMBL3335794) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of recombinant human LDHA in the presence of NADH by resazurin dye reduction method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50362592 (CHEMBL1938870) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mouse SCD-1 | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362592 (CHEMBL1938870) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in Sprague-Dawley rat microsome assessed as reduction in [I-14C] stearoyl CoA desaturation by scintillation counting | J Med Chem 54: 5082-96 (2011) Article DOI: 10.1021/jm200319u BindingDB Entry DOI: 10.7270/Q2348MGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560274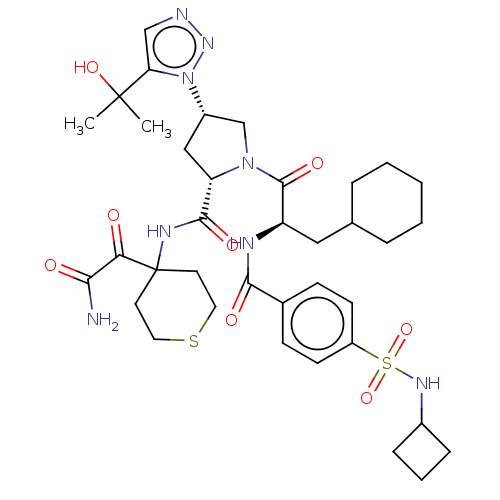 (US11377439, Example 111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50362592 (CHEMBL1938870) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mouse SCD-1 | J Med Chem 54: 5082-96 (2011) Article DOI: 10.1021/jm200319u BindingDB Entry DOI: 10.7270/Q2348MGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362592 (CHEMBL1938870) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of rat SCD-1 | J Med Chem 54: 5082-96 (2011) Article DOI: 10.1021/jm200319u BindingDB Entry DOI: 10.7270/Q2348MGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-Hydroxyacid oxidase 1 (Homo sapiens (Human)) | BDBM50572934 (CHEMBL4847068) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GOX expressed in Escherichia coli C41 (DE3) using glycolate as substrate preincubated for 10 mins followed by substra... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase 5 (Homo sapiens (Human)) | BDBM50364012 (CHEMBL1950397) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human SCD5 | Bioorg Med Chem Lett 21: 7281-6 (2011) Article DOI: 10.1016/j.bmcl.2011.10.040 BindingDB Entry DOI: 10.7270/Q2G44QQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560238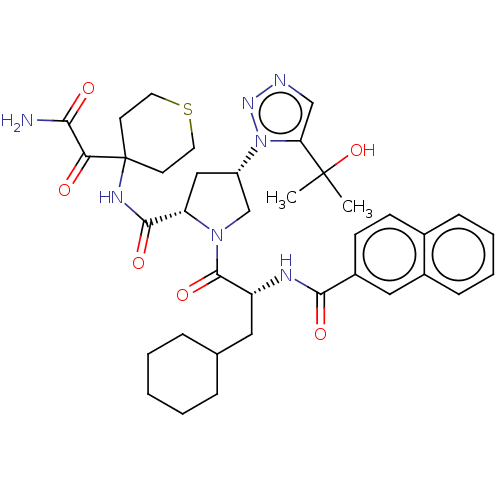 (US11377439, Example 75) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560251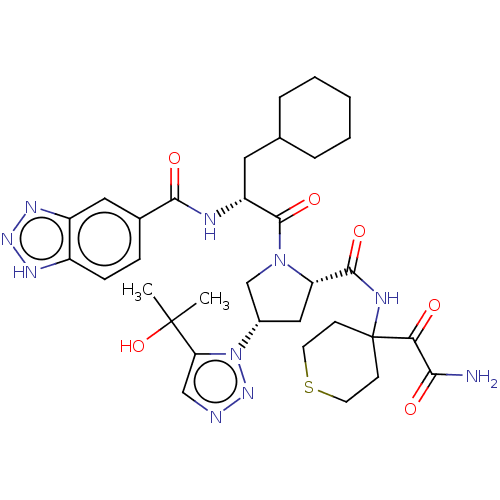 (US11377439, Example 88) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560256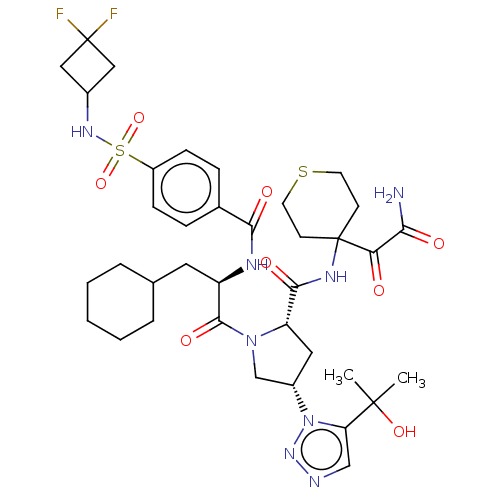 (US11377439, Example 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560266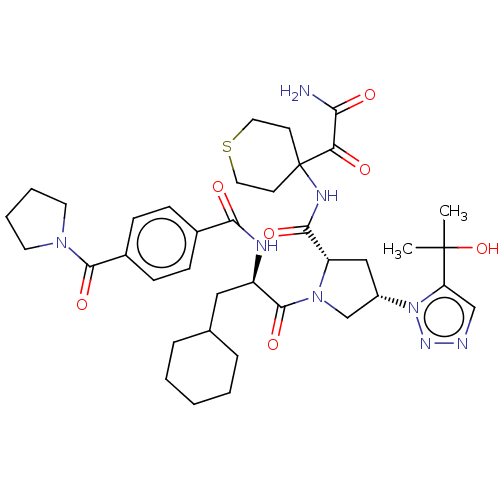 (US11377439, Example 103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560277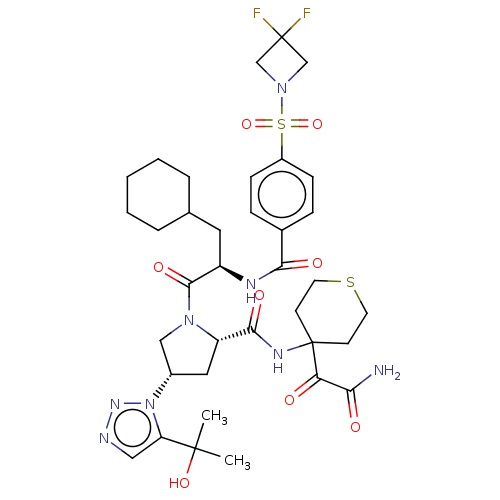 (US11377439, Example 114) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560279 (US11377439, Example 116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560270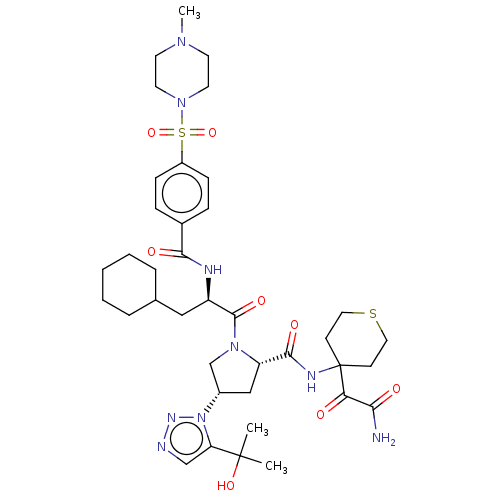 (US11377439, Example 107) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560273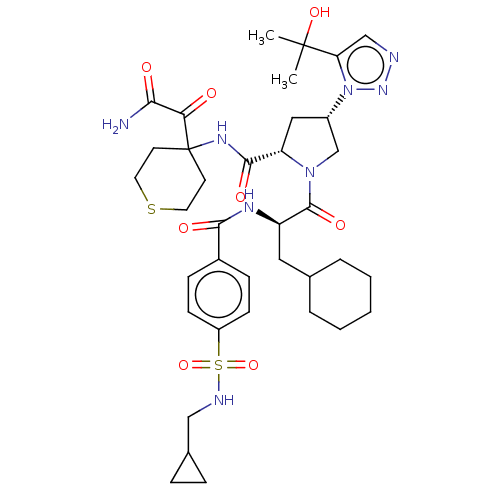 (US11377439, Example 110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560275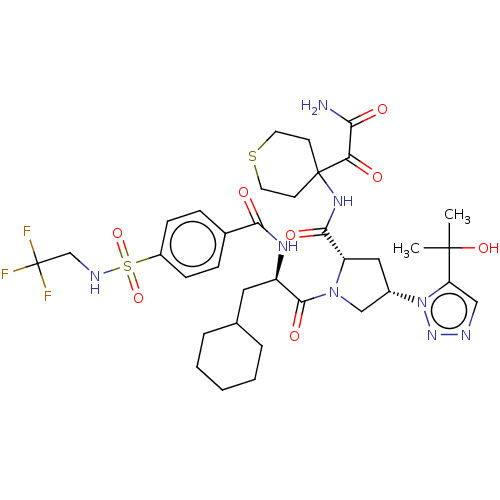 (US11377439, Example 112) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560276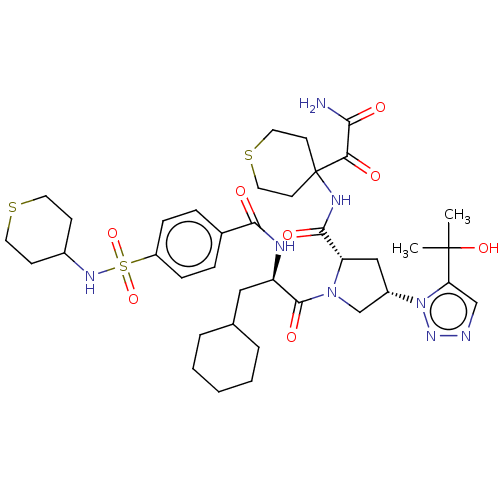 (US11377439, Example 113) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50569643 (CHEMBL4867665) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2-Hydroxyacid oxidase 1 (Homo sapiens (Human)) | BDBM50572929 (CHEMBL4864519) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human GOX expressed in Escherichia coli C41 (DE3) using glycolate as substrate preincubated for 10 mins followed by substra... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00196 BindingDB Entry DOI: 10.7270/Q2V128MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50362592 (CHEMBL1938870) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in human Hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [I-14C] stearoyl CoA de... | J Med Chem 54: 5082-96 (2011) Article DOI: 10.1021/jm200319u BindingDB Entry DOI: 10.7270/Q2348MGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560137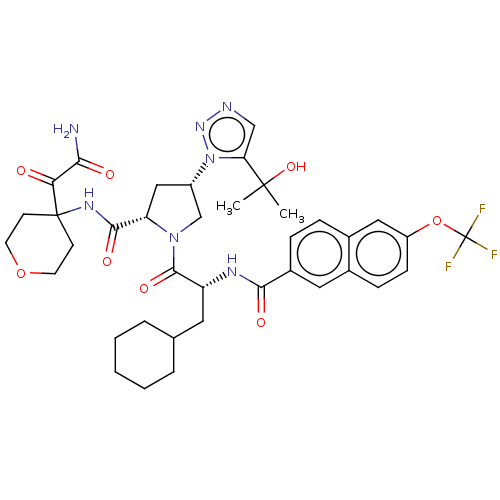 (US11377439, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560255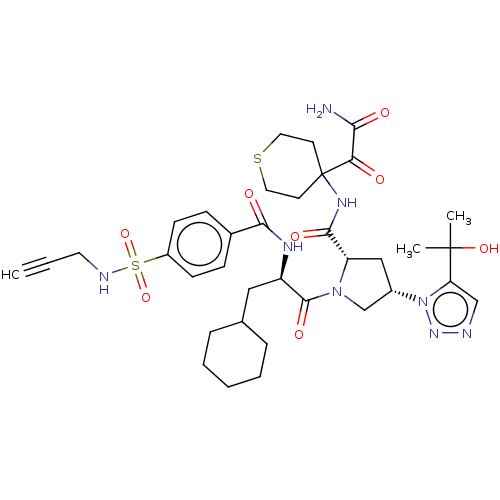 (US11377439, Example 92) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560239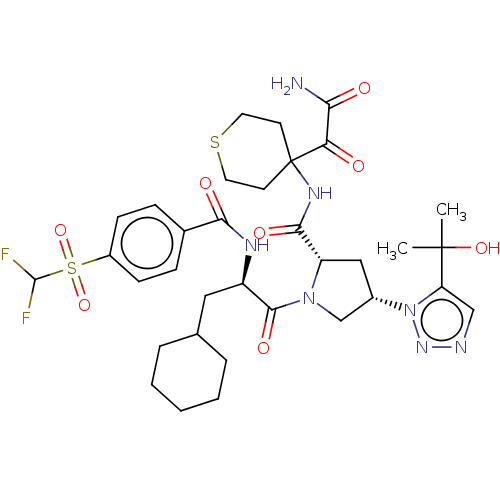 (US11377439, Example 76) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM560246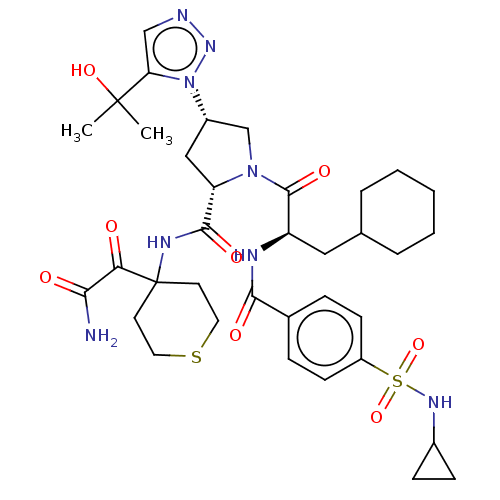 (US11377439, Example 83) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Serial dilutions (1/3) from 1000 μM down to 0.051 μM of test compounds were prepared in dimethyl sulfoxide (DMSO). Then 2 μL of soluti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z03CDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 681 total ) | Next | Last >> |