 Found 64 hits with Last Name = 'sawyer' and Initial = 'da'
Found 64 hits with Last Name = 'sawyer' and Initial = 'da' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Inositol polyphosphate-5-phosphatase A
(Homo sapiens (Human)) | BDBM50279840

((D)-2,2-difluoro-2-deoxy-myo-inositol 1,4,5-tripho...)Show SMILES OC1C(OP([O-])([O-])=O)C(OP([O-])([O-])=O)C(O)C(F)(F)C1OP([O-])([O-])=O Show InChI InChI=1S/C6H13F2O14P3/c7-6(8)4(10)3(21-24(14,15)16)2(20-23(11,12)13)1(9)5(6)22-25(17,18)19/h1-5,9-10H,(H2,11,12,13)(H2,14,15,16)(H2,17,18,19)/p-6 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards Inositol-1,4,5-trisphosphate 5-phosphatase |
Bioorg Med Chem Lett 1: 705-710 (1991)
Article DOI: 10.1016/S0960-894X(01)81052-0
BindingDB Entry DOI: 10.7270/Q2348KV6 |
More data for this
Ligand-Target Pair | |
Inositol 1,4,5-trisphosphate receptor type 1/2/3
(Homo sapiens (Human)) | BDBM50211666
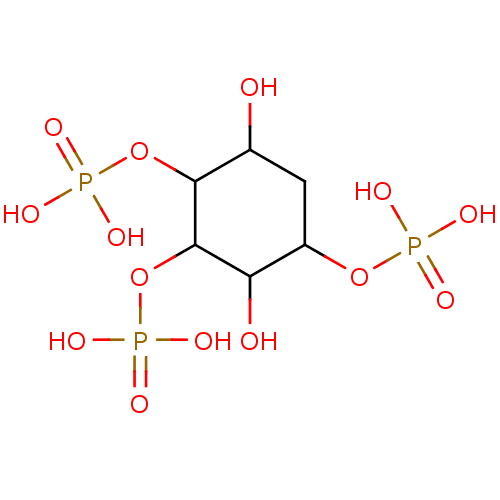
(CHEMBL1161456)Show SMILES OC1CC(OP(O)(O)=O)C(O)C(OP(O)(O)=O)C1OP(O)(O)=O Show InChI InChI=1S/C6H15O14P3/c7-2-1-3(18-21(9,10)11)4(8)6(20-23(15,16)17)5(2)19-22(12,13)14/h2-8H,1H2,(H2,9,10,11)(H2,12,13,14)(H2,15,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 2.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to inhibit Inositol phosphorylation |
Bioorg Med Chem Lett 1: 705-710 (1991)
Article DOI: 10.1016/S0960-894X(01)81052-0
BindingDB Entry DOI: 10.7270/Q2348KV6 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086462

((S)-3-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-p...)Show InChI InChI=1S/C7H15N3O2S/c1-5(8)10-2-3-13-4-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086466

((S)-3-(2-Acetimidoylamino-ethoxy)-2-amino-propioni...)Show InChI InChI=1S/C7H15N3O3/c1-5(8)10-2-3-13-4-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086459

(4-(2-Acetimidoylamino-ethoxy)-2-amino-butyric acid...)Show InChI InChI=1S/C8H17N3O3/c1-6(9)11-3-5-14-4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086467
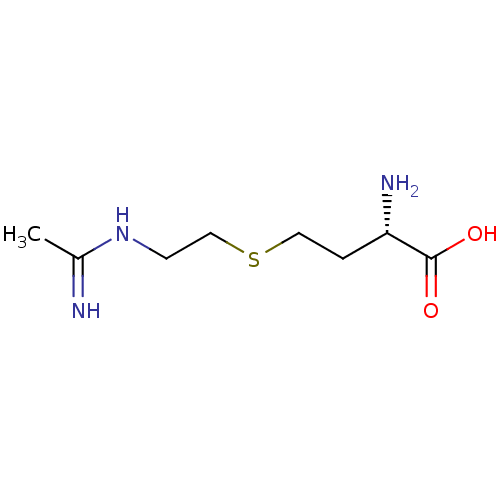
((S)-4-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-b...)Show InChI InChI=1S/C8H17N3O2S/c1-6(9)11-3-5-14-4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50030277
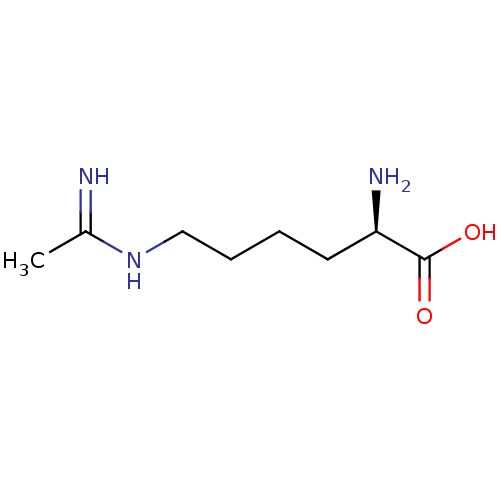
((R)-6-Acetimidoylamino-2-amino-hexanoic acid | CHE...)Show InChI InChI=1S/C8H17N3O2/c1-6(9)11-5-3-2-4-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50035267

(CHEMBL66344 | [2-(4-Decyloxy-3,5-dimethoxy-benzoyl...)Show SMILES CCCCCCCCCCOc1c(OC)cc(cc1OC)C(=O)OCC[N+](C)(C)C Show InChI InChI=1S/C24H42NO5/c1-7-8-9-10-11-12-13-14-16-29-23-21(27-5)18-20(19-22(23)28-6)24(26)30-17-15-25(2,3)4/h18-19H,7-17H2,1-6H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Activity against adenosine diphosphate (ADP) induced platelet aggregation |
J Med Chem 38: 2130-7 (1995)
BindingDB Entry DOI: 10.7270/Q2J38RKC |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50035258

(CHEMBL66185 | [4-(4-Decyloxy-3,5-dimethoxy-benzoyl...)Show SMILES CCCCCCCCCCOc1c(OC)cc(cc1OC)C(=O)OCCCC[N+](C)(C)C Show InChI InChI=1S/C26H46NO5/c1-7-8-9-10-11-12-13-15-18-31-25-23(29-5)20-22(21-24(25)30-6)26(28)32-19-16-14-17-27(2,3)4/h20-21H,7-19H2,1-6H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Activity against adenosine diphosphate (ADP) induced platelet aggregation |
J Med Chem 38: 2130-7 (1995)
BindingDB Entry DOI: 10.7270/Q2J38RKC |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50035259

(CHEMBL67302 | [4-(4-Hexadecyloxy-3,5-dimethoxy-ben...)Show SMILES CCCCCCCCCCCCCCCCOc1c(OC)cc(cc1OC)C(=O)OCCCC[N+](C)(C)C Show InChI InChI=1S/C32H58NO5/c1-7-8-9-10-11-12-13-14-15-16-17-18-19-21-24-37-31-29(35-5)26-28(27-30(31)36-6)32(34)38-25-22-20-23-33(2,3)4/h26-27H,7-25H2,1-6H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Activity against adenosine diphosphate (ADP) induced platelet aggregation |
J Med Chem 38: 2130-7 (1995)
BindingDB Entry DOI: 10.7270/Q2J38RKC |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50230993

((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human endothelial Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50230993

((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086461

((R)-3-(2-acetimidoylamino-ethylsulfanyl)-2-amino-p...)Show InChI InChI=1S/C7H15N3O2S/c1-5(8)10-2-3-13-4-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086470
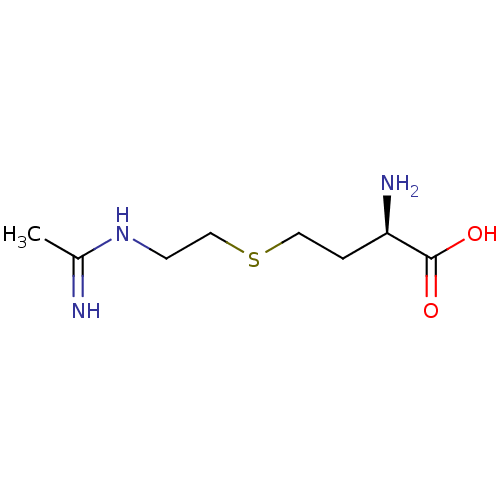
((R)-4-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-b...)Show InChI InChI=1S/C8H17N3O2S/c1-6(9)11-3-5-14-4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50086459

(4-(2-Acetimidoylamino-ethoxy)-2-amino-butyric acid...)Show InChI InChI=1S/C8H17N3O3/c1-6(9)11-3-5-14-4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50230993

((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086468

((S)-3-(2-Acetimidoylamino-ethanesulfinyl)-2-amino-...)Show InChI InChI=1S/C7H15N3O3S/c1-5(8)10-2-3-14(13)4-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-,14?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086464
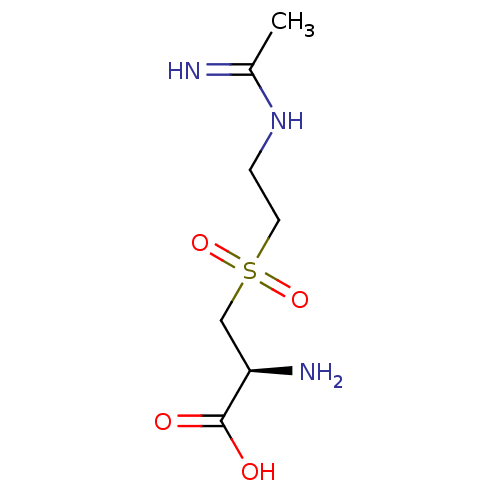
((S)-3-(2-Acetimidoylamino-ethanesulfonyl)-2-amino-...)Show InChI InChI=1S/C7H15N3O4S/c1-5(8)10-2-3-15(13,14)4-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50035262
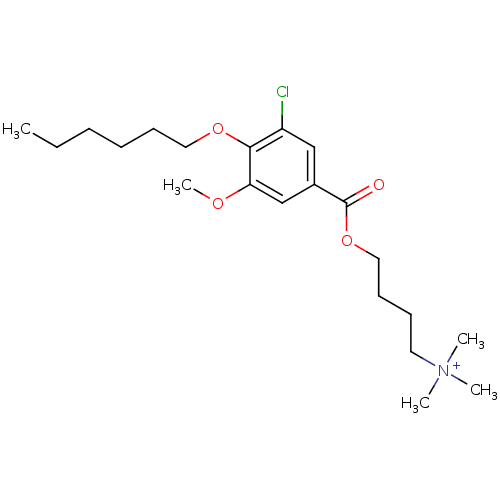
(CHEMBL67232 | [4-(3-Chloro-4-hexyloxy-5-methoxy-be...)Show SMILES CCCCCCOc1c(Cl)cc(cc1OC)C(=O)OCCCC[N+](C)(C)C Show InChI InChI=1S/C21H35ClNO4/c1-6-7-8-10-13-26-20-18(22)15-17(16-19(20)25-5)21(24)27-14-11-9-12-23(2,3)4/h15-16H,6-14H2,1-5H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Activity against adenosine diphosphate (ADP) induced platelet aggregation |
J Med Chem 38: 2130-7 (1995)
BindingDB Entry DOI: 10.7270/Q2J38RKC |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50086466

((S)-3-(2-Acetimidoylamino-ethoxy)-2-amino-propioni...)Show InChI InChI=1S/C7H15N3O3/c1-5(8)10-2-3-13-4-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50035264

(CHEMBL70229 | {2-[4-(4-Hexyloxy-3,5-dimethoxy-benz...)Show SMILES CCCCCCOc1c(OC)cc(cc1OC)C(=O)Oc1ccc(CC[N+](C)(C)C)cc1 Show InChI InChI=1S/C26H38NO5/c1-7-8-9-10-17-31-25-23(29-5)18-21(19-24(25)30-6)26(28)32-22-13-11-20(12-14-22)15-16-27(2,3)4/h11-14,18-19H,7-10,15-17H2,1-6H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Activity against adenosine diphosphate (ADP) induced platelet aggregation |
J Med Chem 38: 2130-7 (1995)
BindingDB Entry DOI: 10.7270/Q2J38RKC |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50035260

(CHEMBL307760 | [4-(4-Hexyloxy-3,5-dimethyl-benzoyl...)Show InChI InChI=1S/C22H38NO3/c1-7-8-9-11-14-25-21-18(2)16-20(17-19(21)3)22(24)26-15-12-10-13-23(4,5)6/h16-17H,7-15H2,1-6H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Activity against adenosine diphosphate (ADP) induced platelet aggregation |
J Med Chem 38: 2130-7 (1995)
BindingDB Entry DOI: 10.7270/Q2J38RKC |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50086466

((S)-3-(2-Acetimidoylamino-ethoxy)-2-amino-propioni...)Show InChI InChI=1S/C7H15N3O3/c1-5(8)10-2-3-13-4-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human endothelial Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50086462

((S)-3-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-p...)Show InChI InChI=1S/C7H15N3O2S/c1-5(8)10-2-3-13-4-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50035266

(5-[4-(4-Hexyloxy-3,5-dimethoxy-phenyl)-butyl]-4-me...)Show InChI InChI=1S/C22H33NO3S/c1-5-6-7-10-13-26-22-19(24-3)14-18(15-20(22)25-4)11-8-9-12-21-17(2)23-16-27-21/h14-16H,5-13H2,1-4H3 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Activity against adenosine diphosphate (ADP) induced platelet aggregation |
J Med Chem 38: 2130-7 (1995)
BindingDB Entry DOI: 10.7270/Q2J38RKC |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50035263
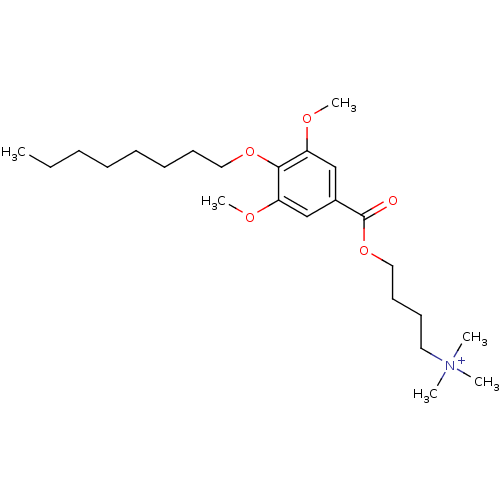
(CHEMBL66234 | [4-(3,5-Dimethoxy-4-octyloxy-benzoyl...)Show SMILES CCCCCCCCOc1c(OC)cc(cc1OC)C(=O)OCCCC[N+](C)(C)C Show InChI InChI=1S/C24H42NO5/c1-7-8-9-10-11-13-16-29-23-21(27-5)18-20(19-22(23)28-6)24(26)30-17-14-12-15-25(2,3)4/h18-19H,7-17H2,1-6H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Activity against adenosine diphosphate (ADP) induced platelet aggregation |
J Med Chem 38: 2130-7 (1995)
BindingDB Entry DOI: 10.7270/Q2J38RKC |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50035265

(CHEMBL67396 | [2-(4-Hexyloxy-3,5-dimethoxy-benzoyl...)Show SMILES CCCCCCOc1c(OC)cc(cc1OC)C(=O)OCc1ccccc1C[N+](C)(C)C Show InChI InChI=1S/C26H38NO5/c1-7-8-9-12-15-31-25-23(29-5)16-22(17-24(25)30-6)26(28)32-19-21-14-11-10-13-20(21)18-27(2,3)4/h10-11,13-14,16-17H,7-9,12,15,18-19H2,1-6H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Activity against adenosine diphosphate (ADP) induced platelet aggregation |
J Med Chem 38: 2130-7 (1995)
BindingDB Entry DOI: 10.7270/Q2J38RKC |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50030276

(7-Acetimidoylamino-2-amino-heptanoic acid | CHEMBL...)Show InChI InChI=1S/C9H19N3O2/c1-7(10)12-6-4-2-3-5-8(11)9(13)14/h8H,2-6,11H2,1H3,(H2,10,12)(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50035257
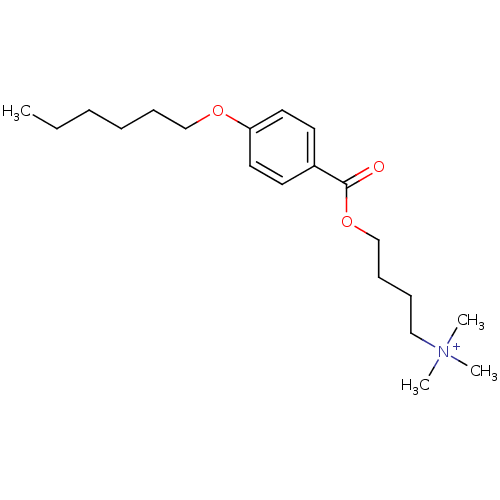
(CHEMBL67031 | [4-(4-Hexyloxy-benzoyloxy)-butyl]-tr...)Show InChI InChI=1S/C20H34NO3/c1-5-6-7-9-16-23-19-13-11-18(12-14-19)20(22)24-17-10-8-15-21(2,3)4/h11-14H,5-10,15-17H2,1-4H3/q+1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Activity against adenosine diphosphate (ADP) induced platelet aggregation |
J Med Chem 38: 2130-7 (1995)
BindingDB Entry DOI: 10.7270/Q2J38RKC |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50030277

((R)-6-Acetimidoylamino-2-amino-hexanoic acid | CHE...)Show InChI InChI=1S/C8H17N3O2/c1-6(9)11-5-3-2-4-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50086462

((S)-3-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-p...)Show InChI InChI=1S/C7H15N3O2S/c1-5(8)10-2-3-13-4-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of endothelial Nitric Oxide Synthase in human |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50086461

((R)-3-(2-acetimidoylamino-ethylsulfanyl)-2-amino-p...)Show InChI InChI=1S/C7H15N3O2S/c1-5(8)10-2-3-13-4-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086465
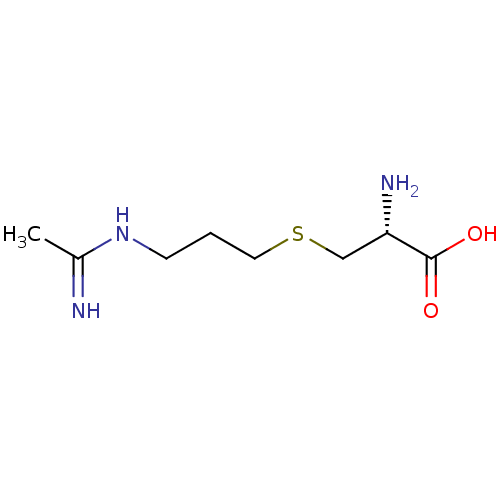
((R)-3-(3-Acetimidoylamino-propylsulfanyl)-2-amino-...)Show InChI InChI=1S/C8H17N3O2S/c1-6(9)11-3-2-4-14-5-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50086468

((S)-3-(2-Acetimidoylamino-ethanesulfinyl)-2-amino-...)Show InChI InChI=1S/C7H15N3O3S/c1-5(8)10-2-3-14(13)4-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50086459

(4-(2-Acetimidoylamino-ethoxy)-2-amino-butyric acid...)Show InChI InChI=1S/C8H17N3O3/c1-6(9)11-3-5-14-4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human endothelial Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50030277

((R)-6-Acetimidoylamino-2-amino-hexanoic acid | CHE...)Show InChI InChI=1S/C8H17N3O2/c1-6(9)11-5-3-2-4-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human endothelial Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50086471
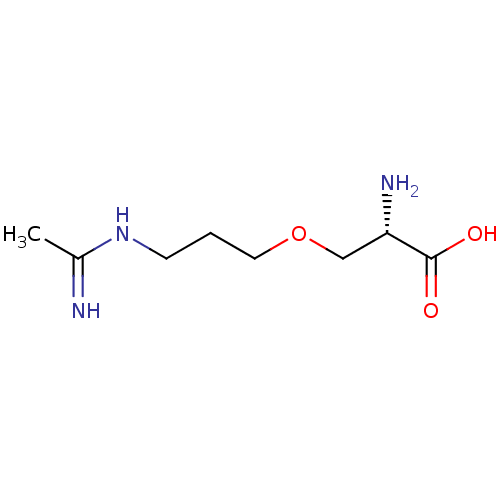
((S)-3-(3-Acetimidoylamino-propoxy)-2-amino-propion...)Show InChI InChI=1S/C8H17N3O3/c1-6(9)11-3-2-4-14-5-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50086463
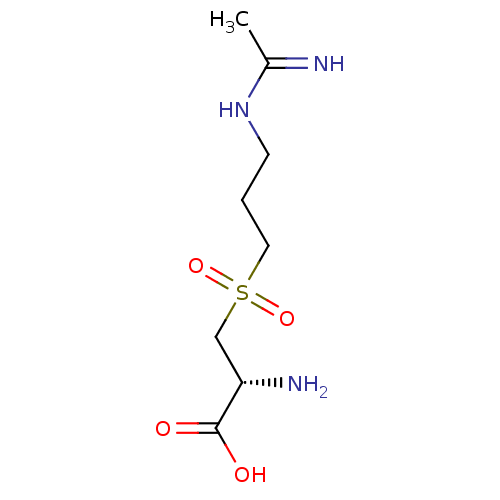
((R)-3-(3-Acetimidoylamino-propane-1-sulfonyl)-2-am...)Show InChI InChI=1S/C8H17N3O4S/c1-6(9)11-3-2-4-16(14,15)5-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human endothelial Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50086465

((R)-3-(3-Acetimidoylamino-propylsulfanyl)-2-amino-...)Show InChI InChI=1S/C8H17N3O2S/c1-6(9)11-3-2-4-14-5-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50086470

((R)-4-(2-Acetimidoylamino-ethylsulfanyl)-2-amino-b...)Show InChI InChI=1S/C8H17N3O2S/c1-6(9)11-3-5-14-4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50086463

((R)-3-(3-Acetimidoylamino-propane-1-sulfonyl)-2-am...)Show InChI InChI=1S/C8H17N3O4S/c1-6(9)11-3-2-4-16(14,15)5-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086471

((S)-3-(3-Acetimidoylamino-propoxy)-2-amino-propion...)Show InChI InChI=1S/C8H17N3O3/c1-6(9)11-3-2-4-14-5-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50086469

(4-(2-Acetimidoylamino-ethanesulfinyl)-2-amino-buty...)Show InChI InChI=1S/C8H17N3O3S/c1-6(9)11-3-5-15(14)4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human endothelial Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50030276

(7-Acetimidoylamino-2-amino-heptanoic acid | CHEMBL...)Show InChI InChI=1S/C9H19N3O2/c1-7(10)12-6-4-2-3-5-8(11)9(13)14/h8H,2-6,11H2,1H3,(H2,10,12)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human neuronal Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50086460

(4-(2-Acetimidoylamino-ethanesulfonyl)-2-amino-buty...)Show InChI InChI=1S/C8H17N3O4S/c1-6(9)11-3-5-16(14,15)4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human endothelial Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50086465

((R)-3-(3-Acetimidoylamino-propylsulfanyl)-2-amino-...)Show InChI InChI=1S/C8H17N3O2S/c1-6(9)11-3-2-4-14-5-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human endothelial Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086463

((R)-3-(3-Acetimidoylamino-propane-1-sulfonyl)-2-am...)Show InChI InChI=1S/C8H17N3O4S/c1-6(9)11-3-2-4-16(14,15)5-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50086460

(4-(2-Acetimidoylamino-ethanesulfonyl)-2-amino-buty...)Show InChI InChI=1S/C8H17N3O4S/c1-6(9)11-3-5-16(14,15)4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of neuronal Nitric Oxide Synthase in human |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50030276

(7-Acetimidoylamino-2-amino-heptanoic acid | CHEMBL...)Show InChI InChI=1S/C9H19N3O2/c1-7(10)12-6-4-2-3-5-8(11)9(13)14/h8H,2-6,11H2,1H3,(H2,10,12)(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human endothelial Nitric Oxide Synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50086469

(4-(2-Acetimidoylamino-ethanesulfinyl)-2-amino-buty...)Show InChI InChI=1S/C8H17N3O3S/c1-6(9)11-3-5-15(14)4-2-7(10)8(12)13/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human inducible nitric oxide synthase |
Bioorg Med Chem Lett 10: 597-600 (2000)
BindingDB Entry DOI: 10.7270/Q21C1XDN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data