

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50423836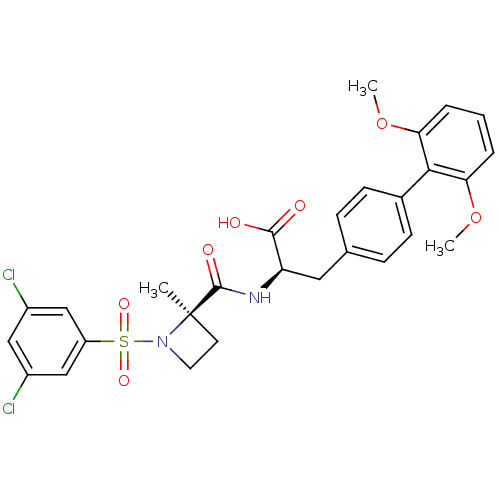 (CHEMBL1829034) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50105400 ((S)-2-{[(S)-2-(3,4-Dimethoxy-benzenesulfonyl)-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50095248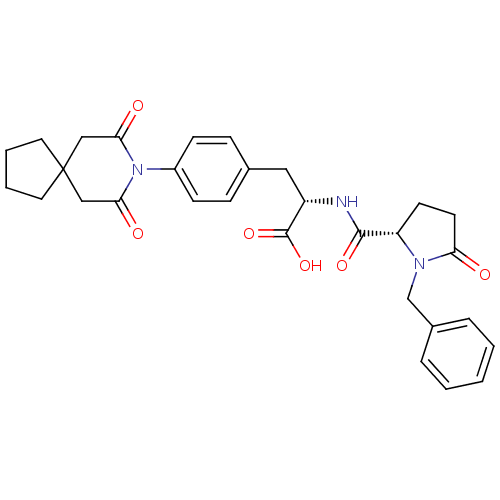 ((S)-2-[((S)-1-Benzyl-5-oxo-pyrrolidine-2-carbonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50423837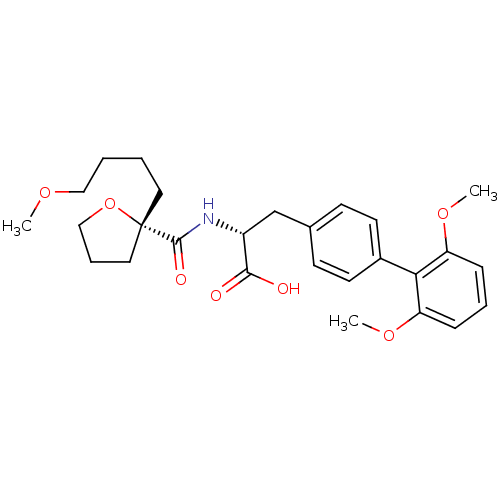 (CHEMBL1829035) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50124286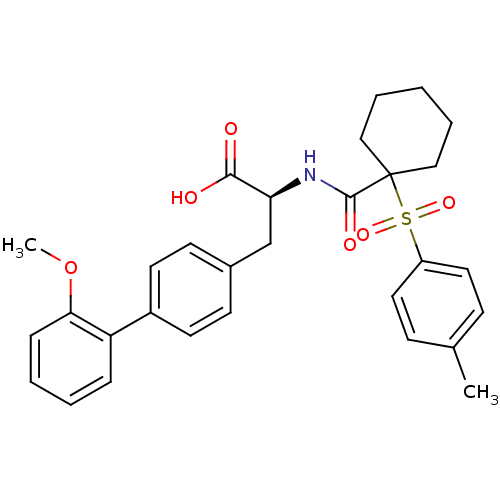 (3-(2'-Methoxy-biphenyl-4-yl)-2-{[1-((S)-toluene-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50423833 (CHEMBL1828842) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50423830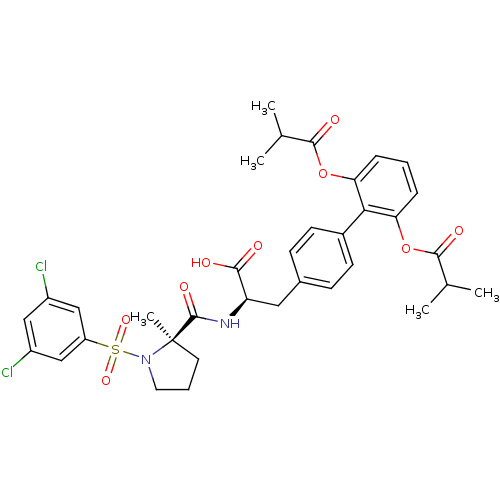 (CHEMBL1828839) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50423832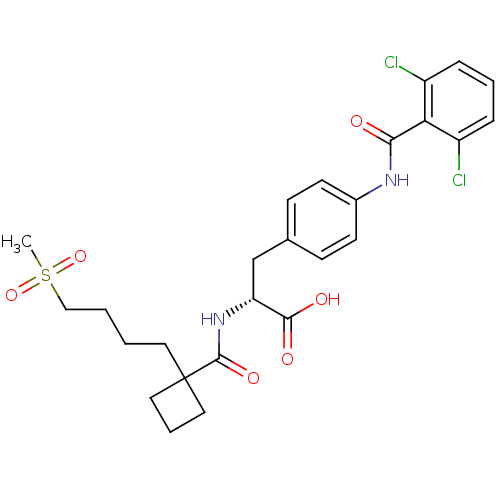 (CHEMBL1828841) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50117056 ((S)-2-(2-Bromo-6-methyl-benzoylamino)-3-[4-(2,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50113545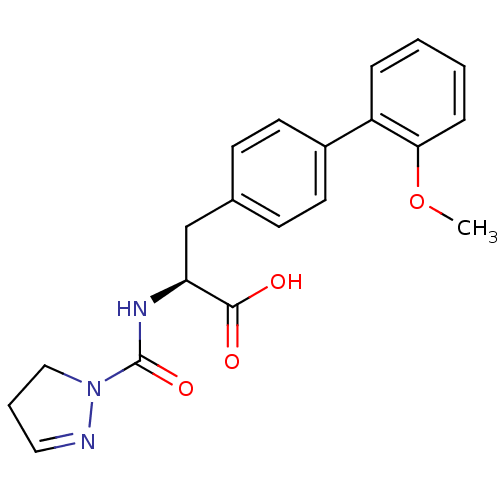 ((S)-2-[(4,5-Dihydro-pyrazole-1-carbonyl)-amino]-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50119087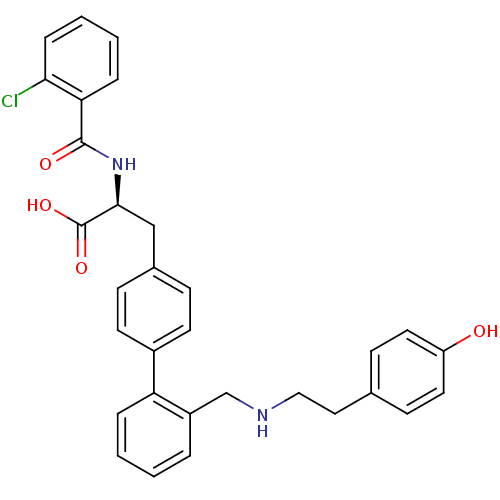 ((S)-2-(2-Chloro-benzoylamino)-3-(2'-{[2-(4-hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50116198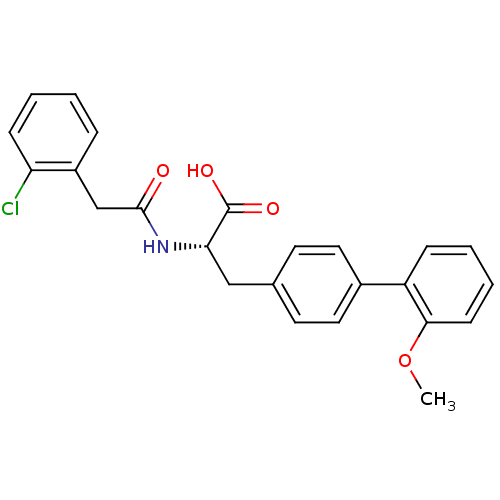 ((S)-2-[2-(2-Chloro-phenyl)-acetylamino]-3-(2'-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50089009 ((S)-2-[((S)-3-Acetyl-thiazolidine-4-carbonyl)-amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50423835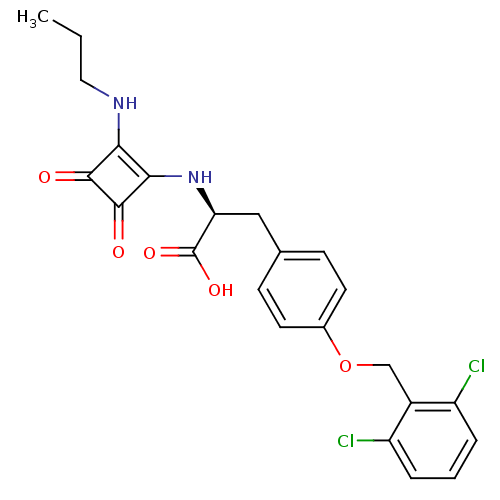 (CHEMBL1829033) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50423834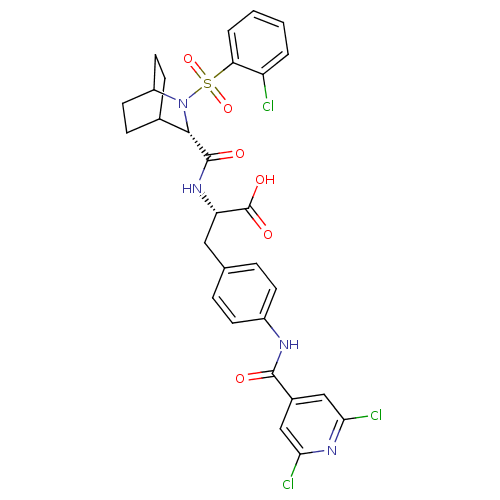 (CHEMBL1829032) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50144767 ((S)-2-{[(S)-1-(3,5-Dichloro-benzenesulfonyl)-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50117076 ((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-[(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2C1 (Rattus norvegicus) | BDBM50367914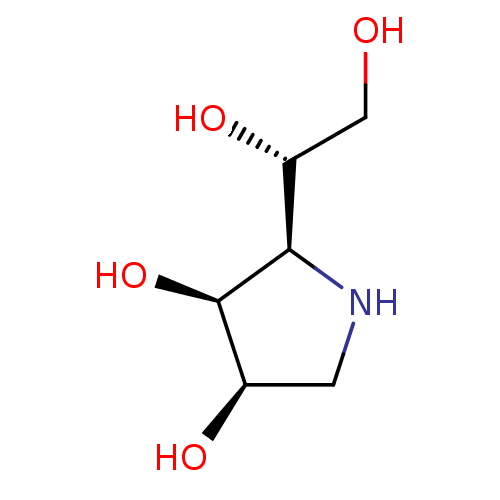 (CHEMBL1794796) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Division of Chemicals and Polymers Curated by ChEMBL | Assay Description Inhibitory activity against Mannosidase in jack bean (Canavalia ensiformis) | J Med Chem 32: 2084-9 (1989) BindingDB Entry DOI: 10.7270/Q2TQ6246 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2C1 (Rattus norvegicus) | BDBM50367915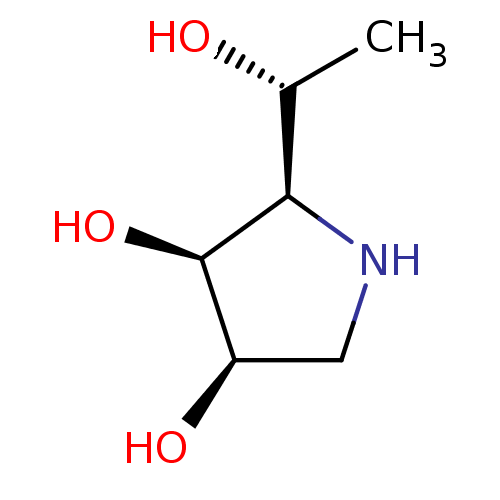 (CHEMBL1794797) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Division of Chemicals and Polymers Curated by ChEMBL | Assay Description Inhibitory activity against Mannosidase in jack bean (Canavalia ensiformis) | J Med Chem 32: 2084-9 (1989) BindingDB Entry DOI: 10.7270/Q2TQ6246 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326776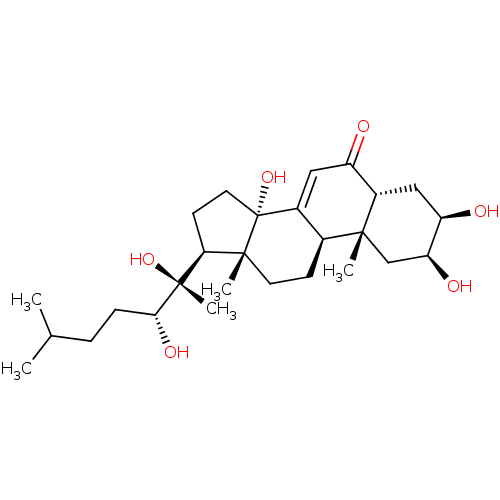 (2,3,14,20,22-PENTAHYDROXYCHOLEST-7-EN-6-ONE | CHEM...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-mannosidase 2C1 (Rattus norvegicus) | BDBM50016706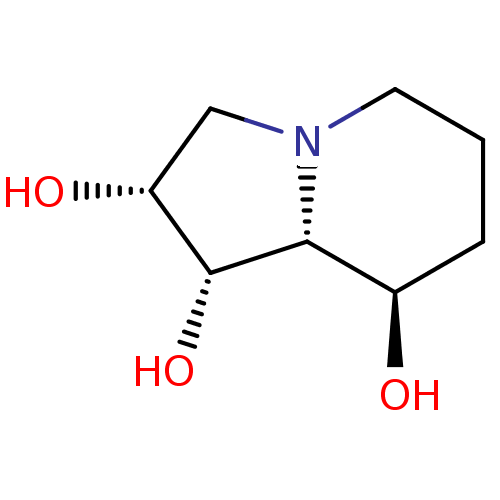 (CHEMBL63139 | Octahydro-indolizine-1,2,8-triol | S...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Division of Chemicals and Polymers Curated by ChEMBL | Assay Description Inhibitory activity against Mannosidase in jack bean (Canavalia ensiformis) | J Med Chem 32: 2084-9 (1989) BindingDB Entry DOI: 10.7270/Q2TQ6246 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50087592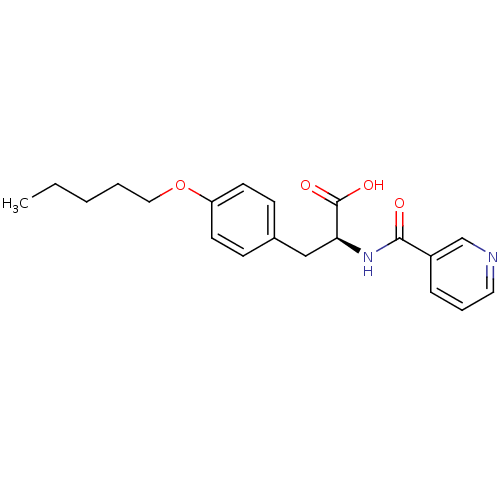 ((S)-3-(4-Pentyloxy-phenyl)-2-[(pyridine-3-carbonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50114294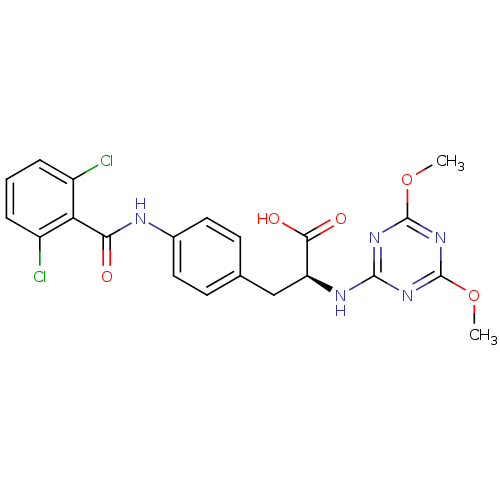 ((S)-3-[4-(2,6-Dichloro-benzoylamino)-phenyl]-2-(4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-4/beta-1 (Homo sapiens (Human)) | BDBM50423831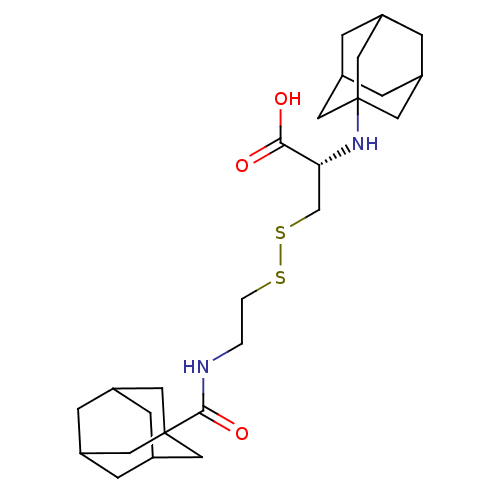 (CHEMBL1828840) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ian Wark Laboratories Curated by ChEMBL | Assay Description Antagonist activity at alpha4beta1 integrin | Bioorg Med Chem 19: 5903-11 (2011) Article DOI: 10.1016/j.bmc.2011.08.011 BindingDB Entry DOI: 10.7270/Q26111MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cadherin-2 (Homo sapiens (Human)) | BDBM50339160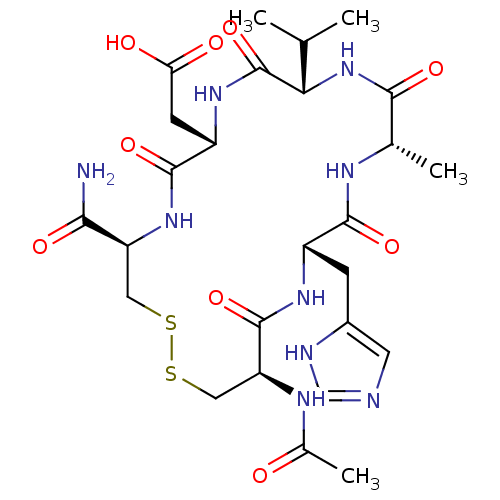 (CHEMBL1689350 | N-Ac-CHAVDC-NH2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering Curated by ChEMBL | Assay Description Antagonist activity at N-cadherin | J Med Chem 54: 1111-25 (2011) Article DOI: 10.1021/jm1012984 BindingDB Entry DOI: 10.7270/Q2QV3MT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326770 (4-isobutyl-5-methylene-2-oxo-1-(2,4,5-trichlorophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326777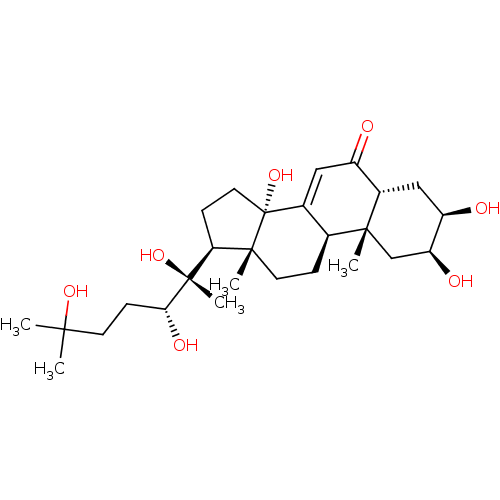 ((2beta,3beta,5beta,22R)-2,3,14,20,22,25-hexahydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326767 (4-butyl-1-(2-tert-butyl-5-chlorophenyl)-5-methylen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326755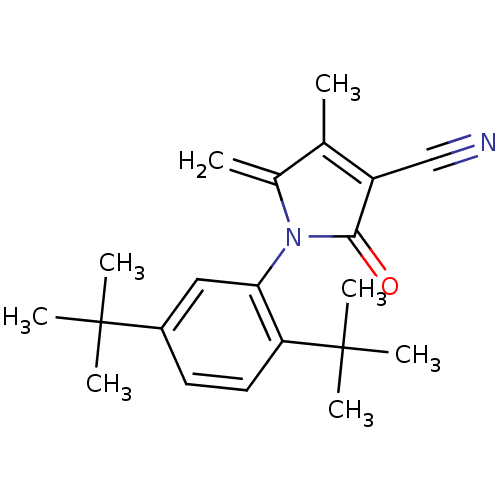 (1-(2,5-di-tert-butylphenyl)-4-methyl-5-methylene-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326760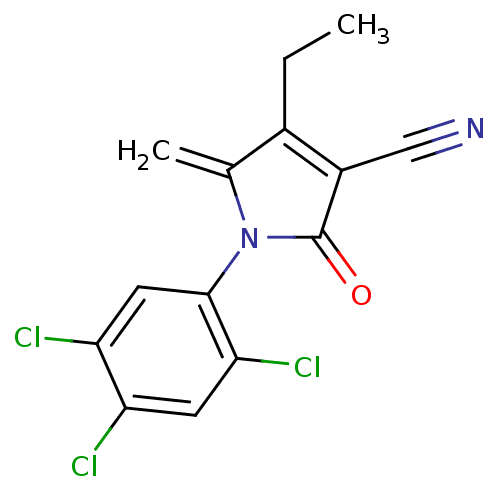 (4-ethyl-5-methylene-2-oxo-1-(2,4,5-trichlorophenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326765 (4-butyl-1-(3-chloro-2-methylphenyl)-5-methylene-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326758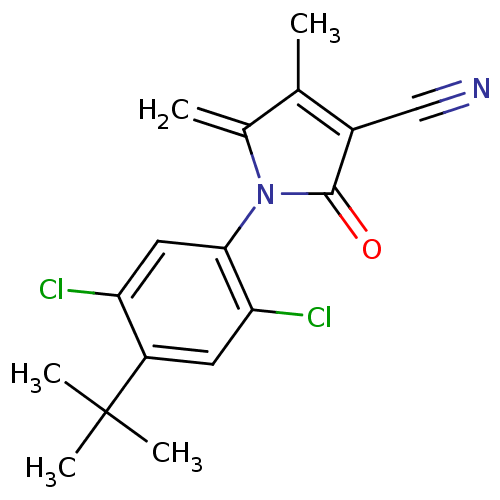 (1-(4-tert-butyl-2,5-dichlorophenyl)-4-methyl-5-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326759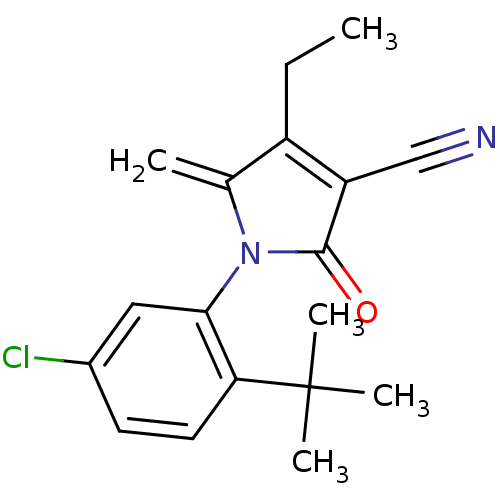 (1-(2-tert-butyl-5-chlorophenyl)-4-ethyl-5-methylen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326766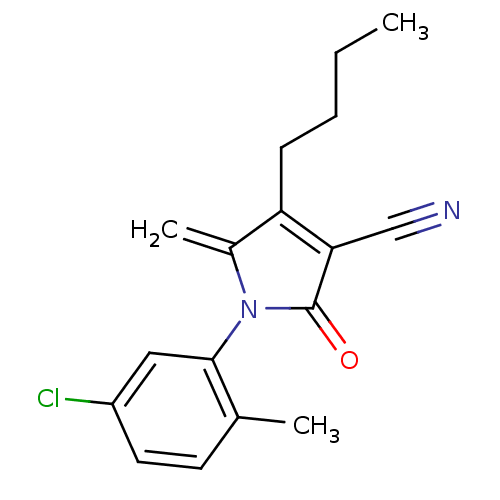 (4-butyl-1-(5-chloro-2-methylphenyl)-5-methylene-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326773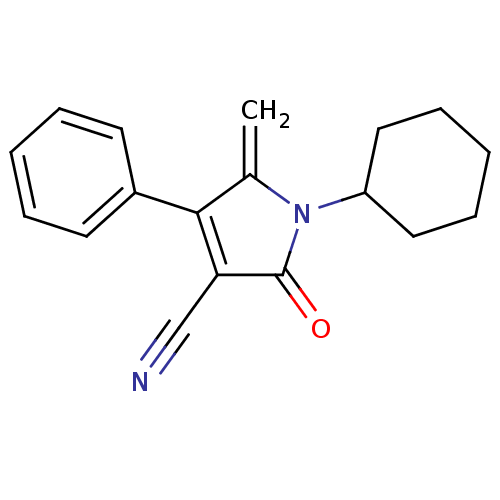 (1-cyclohexyl-5-methylene-2-oxo-4-phenyl-2,5-dihydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326761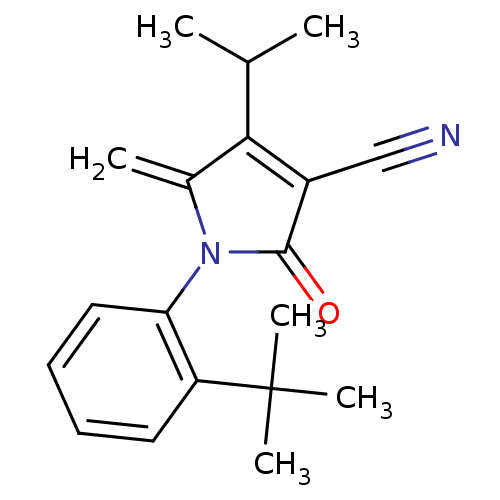 (1-(2-tert-butylphenyl)-4-isopropyl-5-methylene-2-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326774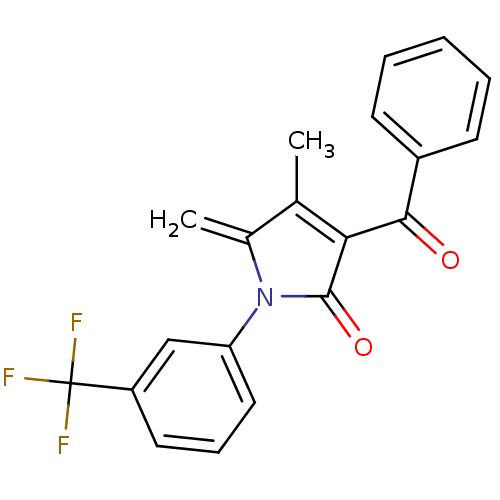 (3-benzoyl-4-methyl-5-methylene-1-(3-(trifluorometh...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326756 (1-(2,6-diisopropylphenyl)-4-methyl-5-methylene-2-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326757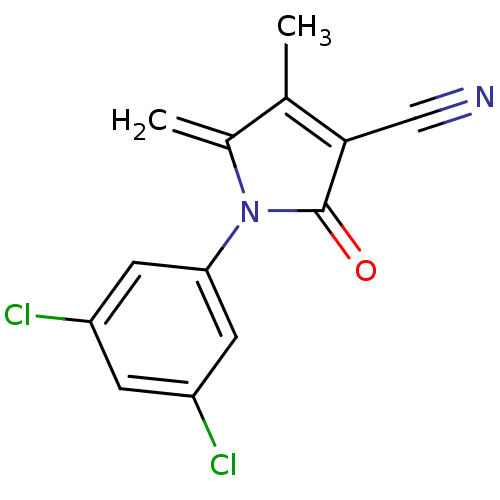 (1-(3,5-dichlorophenyl)-4-methyl-5-methylene-2-oxo-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326769 (4-butyl-1-(2-ethyl-6-methylphenyl)-5-methylene-2-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326762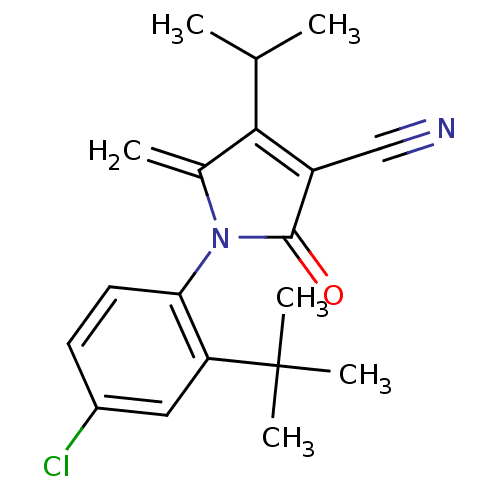 (1-(2-tert-butyl-4-chlorophenyl)-4-isopropyl-5-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326763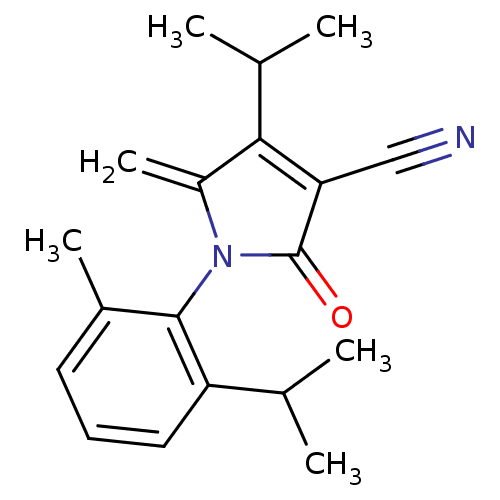 (4-isopropyl-1-(2-isopropyl-6-methylphenyl)-5-methy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326754 (4-methyl-5-methylene-2-oxo-1-(2-(trifluoromethyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326772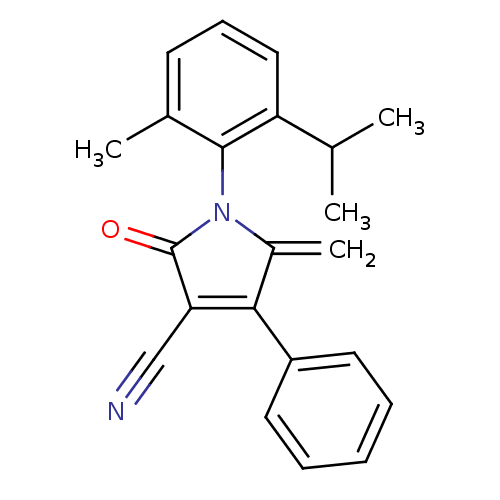 (1-(2-isopropyl-6-methylphenyl)-5-methylene-2-oxo-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326768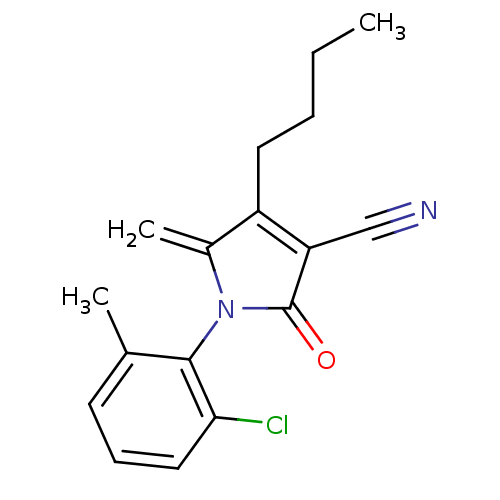 (4-butyl-1-(2-chloro-6-methylphenyl)-5-methylene-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2C1 (Rattus norvegicus) | BDBM50016703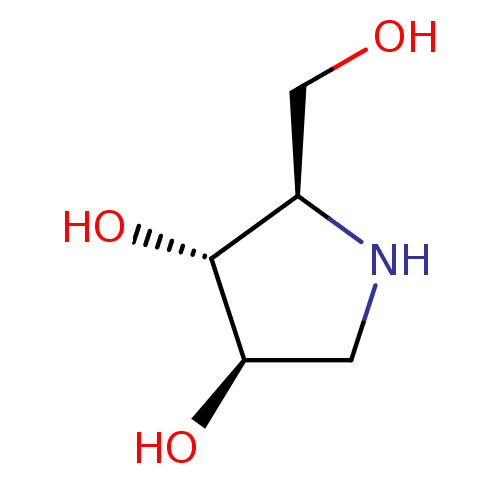 (2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Division of Chemicals and Polymers Curated by ChEMBL | Assay Description Inhibitory activity against Mannosidase in jack bean (Canavalia ensiformis) | J Med Chem 32: 2084-9 (1989) BindingDB Entry DOI: 10.7270/Q2TQ6246 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2C1 (Rattus norvegicus) | BDBM50403114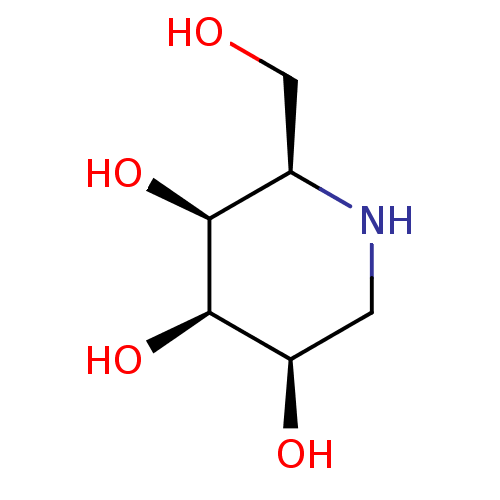 (CHEMBL1337303) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Division of Chemicals and Polymers Curated by ChEMBL | Assay Description Inhibitory activity against Mannosidase in jack bean (Canavalia ensiformis) | J Med Chem 32: 2084-9 (1989) BindingDB Entry DOI: 10.7270/Q2TQ6246 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326771 (4-isobutyl-1-mesityl-5-methylene-2-oxo-2,5-dihydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326775 (CHEMBL1254537 | N,1-Bis(3-chlorophenyl)-4-methyl-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Lucilia cuprina) | BDBM50326764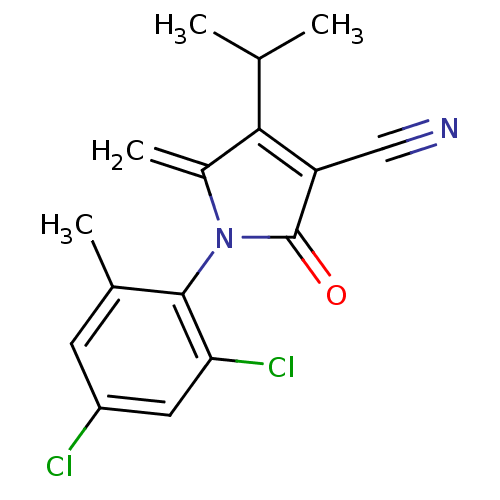 (1-(2,4-dichloro-6-methylphenyl)-4-isopropyl-5-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Molecular and Health Technologies Curated by ChEMBL | Assay Description Binding affinity to Lucilia cuprina recombinant ecdysone receptor ligand binding domain after 3 hrs by fluorescence polarization assay | Bioorg Med Chem 18: 5647-60 (2010) Article DOI: 10.1016/j.bmc.2010.06.020 BindingDB Entry DOI: 10.7270/Q2MK6D43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |