 Found 265 hits with Last Name = 'brockstedt' and Initial = 'dg'
Found 265 hits with Last Name = 'brockstedt' and Initial = 'dg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538576
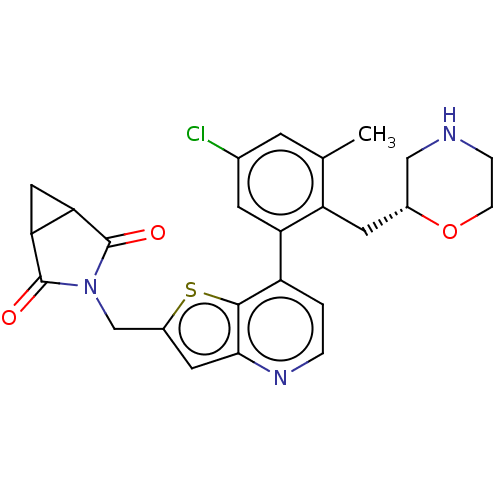
(CHEMBL4634092)Show SMILES Cc1cc(Cl)cc(c1C[C@@H]1CNCCO1)-c1ccnc2cc(CN3C(=O)C4CC4C3=O)sc12 |r| Show InChI InChI=1S/C25H24ClN3O3S/c1-13-6-14(26)7-19(18(13)8-15-11-27-4-5-32-15)17-2-3-28-22-9-16(33-23(17)22)12-29-24(30)20-10-21(20)25(29)31/h2-3,6-7,9,15,20-21,27H,4-5,8,10-12H2,1H3/t15-,20?,21?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538560

(CHEMBL4640002)Show SMILES Clc1cc2C[C@@H](Oc2c(c1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12)C(=O)N1CCNCC1 |r| Show InChI InChI=1S/C25H23ClN4O4S/c26-15-9-14-10-20(25(33)29-7-5-27-6-8-29)34-23(14)18(11-15)17-3-4-28-19-12-16(35-24(17)19)13-30-21(31)1-2-22(30)32/h3-4,9,11-12,20,27H,1-2,5-8,10,13H2/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538571
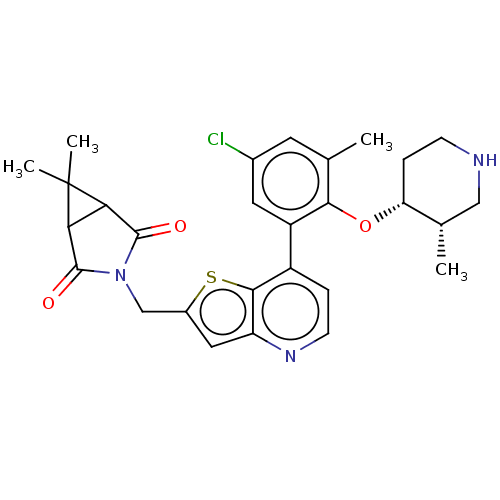
(CHEMBL4635160)Show SMILES C[C@H]1CNCC[C@H]1Oc1c(C)cc(Cl)cc1-c1ccnc2cc(CN3C(=O)C4C(C3=O)C4(C)C)sc12 |r| Show InChI InChI=1S/C28H30ClN3O3S/c1-14-9-16(29)10-19(24(14)35-21-6-7-30-12-15(21)2)18-5-8-31-20-11-17(36-25(18)20)13-32-26(33)22-23(27(32)34)28(22,3)4/h5,8-11,15,21-23,30H,6-7,12-13H2,1-4H3/t15-,21+,22?,23?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538572

(CHEMBL4649132)Show SMILES C[C@H]1CNCC[C@H]1Oc1c(C)cc(Cl)cc1-c1ccnc2cc(Cn3c(=O)ccn(CC(F)(F)F)c3=O)sc12 |r| Show InChI InChI=1S/C27H26ClF3N4O3S/c1-15-9-17(28)10-20(24(15)38-22-4-6-32-12-16(22)2)19-3-7-33-21-11-18(39-25(19)21)13-35-23(36)5-8-34(26(35)37)14-27(29,30)31/h3,5,7-11,16,22,32H,4,6,12-14H2,1-2H3/t16-,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538575
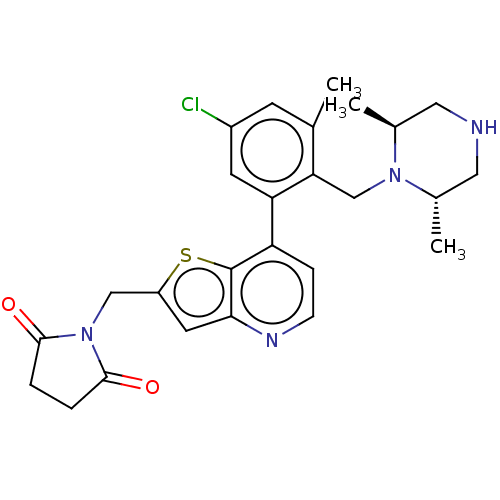
(CHEMBL4638458)Show SMILES C[C@H]1CNC[C@H](C)N1Cc1c(C)cc(Cl)cc1-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 |r| Show InChI InChI=1S/C26H29ClN4O2S/c1-15-8-18(27)9-21(22(15)14-30-16(2)11-28-12-17(30)3)20-6-7-29-23-10-19(34-26(20)23)13-31-24(32)4-5-25(31)33/h6-10,16-17,28H,4-5,11-14H2,1-3H3/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538562

(CHEMBL4643330)Show SMILES Clc1cc2C[C@@H](Oc2c(c1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12)c1onc2CCNCc12 |r| Show InChI InChI=1S/C26H21ClN4O4S/c27-14-7-13-8-21(25-18-11-28-5-4-19(18)30-35-25)34-24(13)17(9-14)16-3-6-29-20-10-15(36-26(16)20)12-31-22(32)1-2-23(31)33/h3,6-7,9-10,21,28H,1-2,4-5,8,11-12H2/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538578
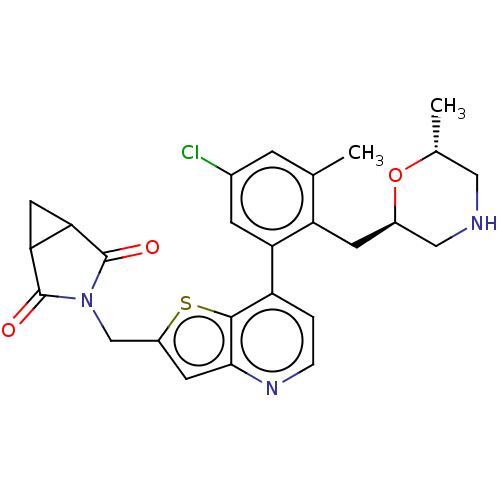
(CHEMBL4640729)Show SMILES C[C@@H]1CNC[C@@H](Cc2c(C)cc(Cl)cc2-c2ccnc3cc(CN4C(=O)C5CC5C4=O)sc23)O1 |r| Show InChI InChI=1S/C26H26ClN3O3S/c1-13-5-15(27)6-20(19(13)7-16-11-28-10-14(2)33-16)18-3-4-29-23-8-17(34-24(18)23)12-30-25(31)21-9-22(21)26(30)32/h3-6,8,14,16,21-22,28H,7,9-12H2,1-2H3/t14-,16-,21?,22?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538579

(CHEMBL4647217)Show SMILES Cc1cc(Cl)cc(c1C[C@@H]1CNCCO1)-c1ccnc2cc(CN3C(=O)C4C(C3=O)C4(C)C)sc12 |r| Show InChI InChI=1S/C27H28ClN3O3S/c1-14-8-15(28)9-20(19(14)10-16-12-29-6-7-34-16)18-4-5-30-21-11-17(35-24(18)21)13-31-25(32)22-23(26(31)33)27(22,2)3/h4-5,8-9,11,16,22-23,29H,6-7,10,12-13H2,1-3H3/t16-,22?,23?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538582
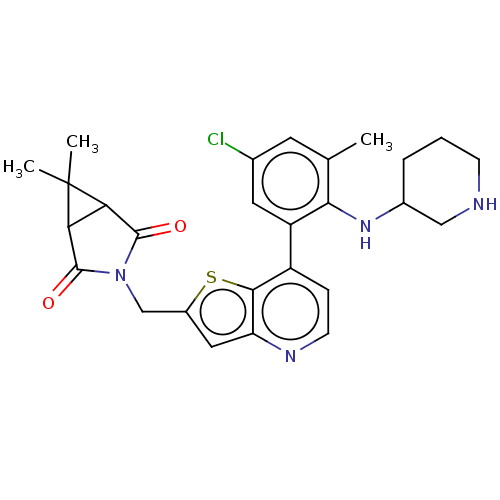
(CHEMBL4638998)Show SMILES Cc1cc(Cl)cc(c1NC1CCCNC1)-c1ccnc2cc(CN3C(=O)C4C(C3=O)C4(C)C)sc12 Show InChI InChI=1S/C27H29ClN4O2S/c1-14-9-15(28)10-19(23(14)31-16-5-4-7-29-12-16)18-6-8-30-20-11-17(35-24(18)20)13-32-25(33)21-22(26(32)34)27(21,2)3/h6,8-11,16,21-22,29,31H,4-5,7,12-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538563

(CHEMBL4648116)Show SMILES Cn1nc2CCNCc2c1[C@H]1Cc2cc(Cl)cc(c2O1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 |r| Show InChI InChI=1S/C27H24ClN5O3S/c1-32-25(19-12-29-6-5-20(19)31-32)22-9-14-8-15(28)10-18(26(14)36-22)17-4-7-30-21-11-16(37-27(17)21)13-33-23(34)2-3-24(33)35/h4,7-8,10-11,22,29H,2-3,5-6,9,12-13H2,1H3/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538570

(CHEMBL4638573)Show SMILES C[C@H]1CNCC[C@H]1Oc1c(C)cc(Cl)cc1-c1ccnc2cc(CN3C(=O)C4CC4C3=O)sc12 |r| Show InChI InChI=1S/C26H26ClN3O3S/c1-13-7-15(27)8-18(23(13)33-22-4-5-28-11-14(22)2)17-3-6-29-21-9-16(34-24(17)21)12-30-25(31)19-10-20(19)26(30)32/h3,6-9,14,19-20,22,28H,4-5,10-12H2,1-2H3/t14-,19?,20?,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538564
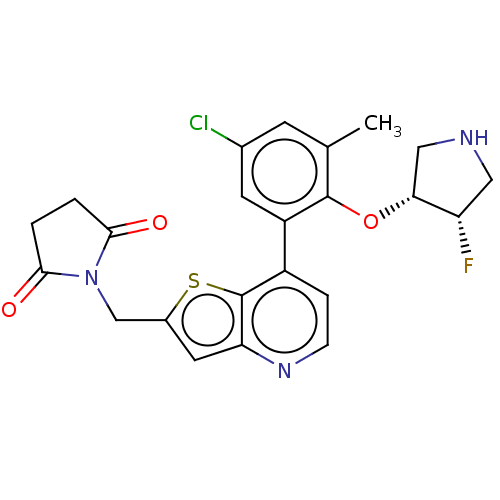
(CHEMBL4641569)Show SMILES Cc1cc(Cl)cc(c1O[C@@H]1CNC[C@@H]1F)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 |r| Show InChI InChI=1S/C23H21ClFN3O3S/c1-12-6-13(24)7-16(22(12)31-19-10-26-9-17(19)25)15-4-5-27-18-8-14(32-23(15)18)11-28-20(29)2-3-21(28)30/h4-8,17,19,26H,2-3,9-11H2,1H3/t17-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538583

(CHEMBL4643112)Show SMILES Cc1cc(Cl)cc(c1CC1CCCNC1)-c1ccnc2cc(CN3C(=O)C4C(C3=O)C4(C)C)sc12 Show InChI InChI=1S/C28H30ClN3O2S/c1-15-9-17(29)11-21(20(15)10-16-5-4-7-30-13-16)19-6-8-31-22-12-18(35-25(19)22)14-32-26(33)23-24(27(32)34)28(23,2)3/h6,8-9,11-12,16,23-24,30H,4-5,7,10,13-14H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538577
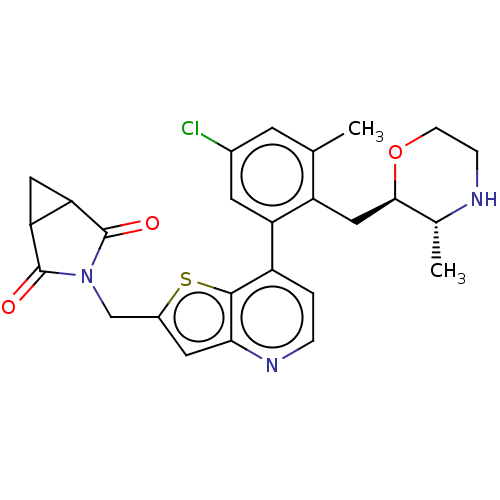
(CHEMBL4648623)Show SMILES C[C@H]1NCCO[C@@H]1Cc1c(C)cc(Cl)cc1-c1ccnc2cc(CN3C(=O)C4CC4C3=O)sc12 |r| Show InChI InChI=1S/C26H26ClN3O3S/c1-13-7-15(27)8-19(18(13)11-23-14(2)28-5-6-33-23)17-3-4-29-22-9-16(34-24(17)22)12-30-25(31)20-10-21(20)26(30)32/h3-4,7-9,14,20-21,23,28H,5-6,10-12H2,1-2H3/t14-,20?,21?,23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538567
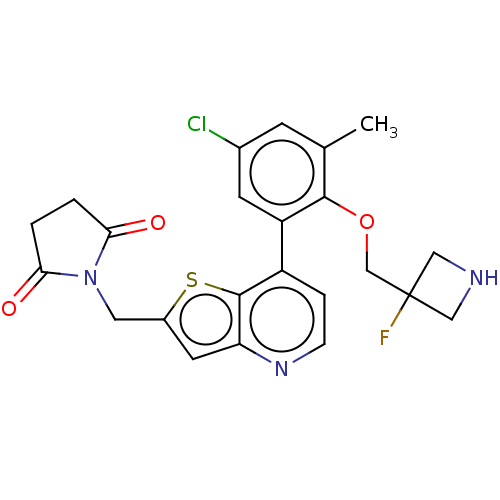
(CHEMBL4633860)Show SMILES Cc1cc(Cl)cc(c1OCC1(F)CNC1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 Show InChI InChI=1S/C23H21ClFN3O3S/c1-13-6-14(24)7-17(21(13)31-12-23(25)10-26-11-23)16-4-5-27-18-8-15(32-22(16)18)9-28-19(29)2-3-20(28)30/h4-8,26H,2-3,9-12H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591075
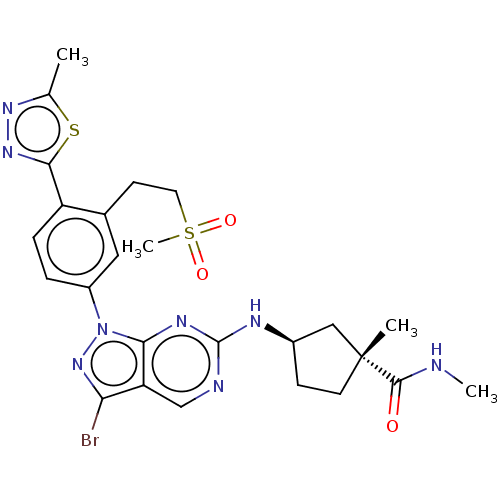
(CHEMBL5200118)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(CCS(C)(=O)=O)c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538569

(CHEMBL4644317)Show SMILES C[C@H]1CNCC[C@H]1Oc1c(C)cc(Cl)cc1-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 |r| Show InChI InChI=1S/C25H26ClN3O3S/c1-14-9-16(26)10-19(24(14)32-21-6-7-27-12-15(21)2)18-5-8-28-20-11-17(33-25(18)20)13-29-22(30)3-4-23(29)31/h5,8-11,15,21,27H,3-4,6-7,12-13H2,1-2H3/t15-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538581
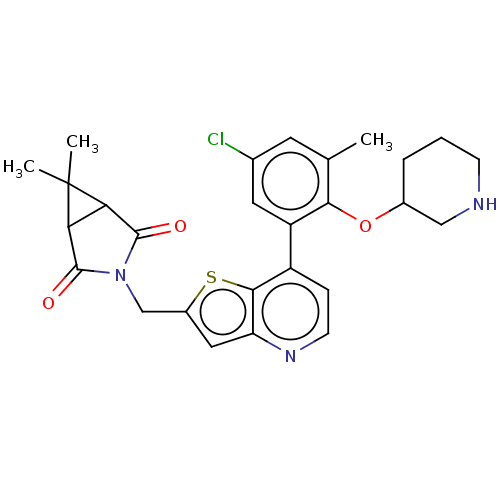
(CHEMBL4642862)Show SMILES Cc1cc(Cl)cc(c1OC1CCCNC1)-c1ccnc2cc(CN3C(=O)C4C(C3=O)C4(C)C)sc12 Show InChI InChI=1S/C27H28ClN3O3S/c1-14-9-15(28)10-19(23(14)34-16-5-4-7-29-12-16)18-6-8-30-20-11-17(35-24(18)20)13-31-25(32)21-22(26(31)33)27(21,2)3/h6,8-11,16,21-22,29H,4-5,7,12-13H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591046
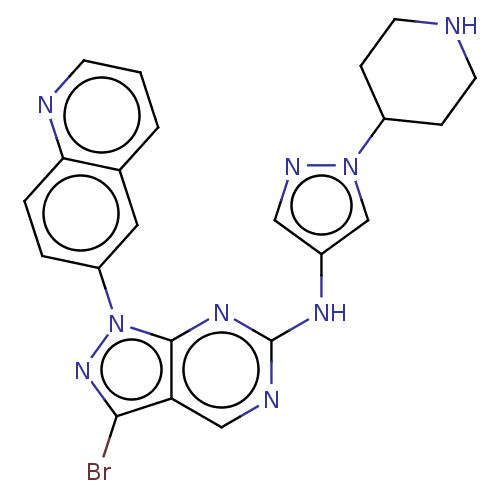
(CHEMBL5202342)Show SMILES Brc1nn(-c2ccc3ncccc3c2)c2nc(Nc3cnn(c3)C3CCNCC3)ncc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538568
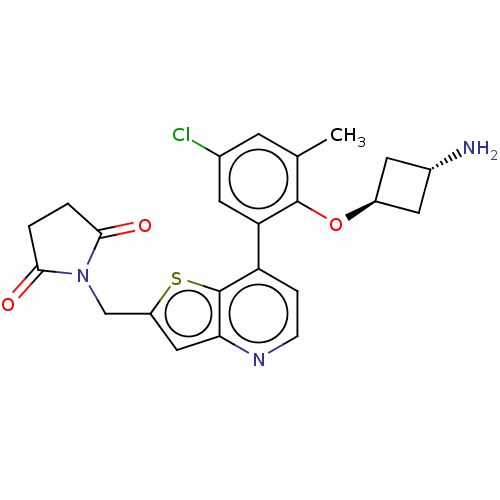
(CHEMBL4640563)Show SMILES Cc1cc(Cl)cc(c1O[C@H]1C[C@H](N)C1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 |r,wU:9.9,wD:11.12,(39.39,-38.53,;38.06,-37.76,;36.71,-38.54,;35.38,-37.77,;34.03,-38.54,;35.38,-36.22,;36.71,-35.44,;38.05,-36.21,;39.38,-35.44,;40.72,-36.2,;42.2,-35.79,;42.6,-37.28,;43.94,-38.04,;41.12,-37.68,;36.7,-33.9,;38.04,-33.13,;38.04,-31.58,;36.69,-30.81,;35.35,-31.6,;33.88,-31.14,;32.99,-32.39,;31.45,-32.4,;30.69,-33.74,;29.16,-33.91,;28.12,-32.78,;28.85,-35.42,;30.19,-36.18,;31.33,-35.14,;32.84,-35.45,;33.91,-33.62,;35.36,-33.14,)| Show InChI InChI=1S/C23H22ClN3O3S/c1-12-6-13(24)7-18(22(12)30-15-8-14(25)9-15)17-4-5-26-19-10-16(31-23(17)19)11-27-20(28)2-3-21(27)29/h4-7,10,14-15H,2-3,8-9,11,25H2,1H3/t14-,15- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538565
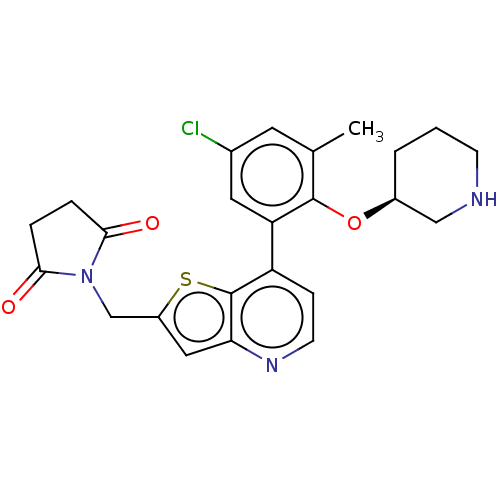
(CHEMBL4635798)Show SMILES Cc1cc(Cl)cc(c1O[C@H]1CCCNC1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 |r| Show InChI InChI=1S/C24H24ClN3O3S/c1-14-9-15(25)10-19(23(14)31-16-3-2-7-26-12-16)18-6-8-27-20-11-17(32-24(18)20)13-28-21(29)4-5-22(28)30/h6,8-11,16,26H,2-5,7,12-13H2,1H3/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591066
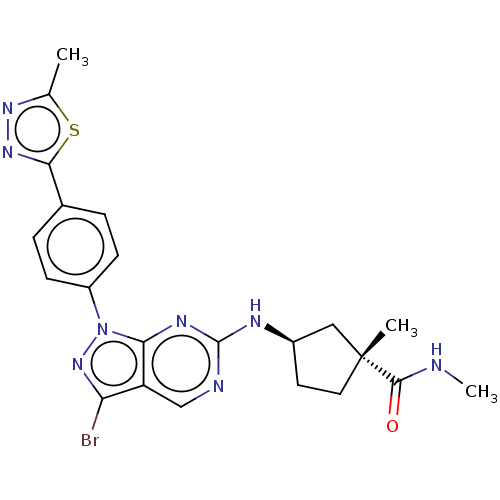
(CHEMBL5190023)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(cc3)-c3nnc(C)s3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591054
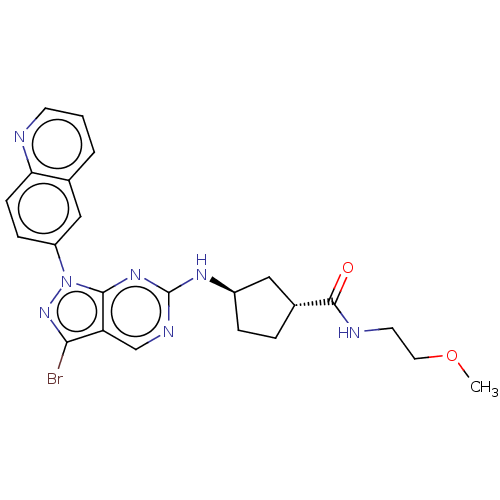
(CHEMBL5179922)Show SMILES COCCNC(=O)[C@@H]1CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc4ncccc4c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591045

(CHEMBL5209076)Show SMILES Clc1nn(-c2ccc3ncccc3c2)c2nc(Nc3cnn(c3)C3CCNCC3)ncc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50545768
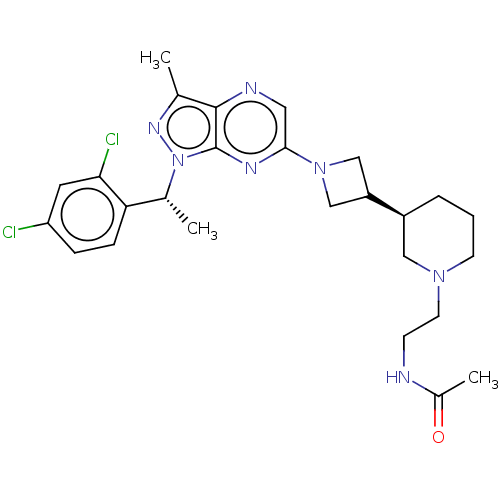
(CHEMBL4641127)Show SMILES C[C@H](c1ccc(Cl)cc1Cl)n1nc(C)c2ncc(nc12)N1CC(C1)[C@H]1CCCN(CCNC(C)=O)C1 |r| Show InChI InChI=1S/C26H33Cl2N7O/c1-16-25-26(35(32-16)17(2)22-7-6-21(27)11-23(22)28)31-24(12-30-25)34-14-20(15-34)19-5-4-9-33(13-19)10-8-29-18(3)36/h6-7,11-12,17,19-20H,4-5,8-10,13-15H2,1-3H3,(H,29,36)/t17-,19+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... |
J Med Chem 63: 8584-8607 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00988
BindingDB Entry DOI: 10.7270/Q2DZ0CW6 |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538574

(CHEMBL4638688)Show SMILES C[C@H]1CNCCN1Cc1c(C)cc(Cl)cc1-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 |r| Show InChI InChI=1S/C25H27ClN4O2S/c1-15-9-17(26)10-20(21(15)14-29-8-7-27-12-16(29)2)19-5-6-28-22-11-18(33-25(19)22)13-30-23(31)3-4-24(30)32/h5-6,9-11,16,27H,3-4,7-8,12-14H2,1-2H3/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591070
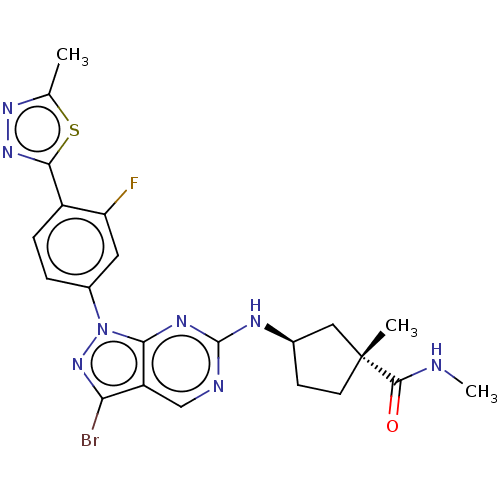
(CHEMBL5208600)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(F)c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50545779

(CHEMBL4642563)Show SMILES C[C@H](c1ccc(Cl)cc1Cl)n1nc(C(N)=O)c2ncc(nc12)N1CC(C1)[C@H]1CCCN(CCO)C1 |r| Show InChI InChI=1S/C24H29Cl2N7O2/c1-14(18-5-4-17(25)9-19(18)26)33-24-22(21(30-33)23(27)35)28-10-20(29-24)32-12-16(13-32)15-3-2-6-31(11-15)7-8-34/h4-5,9-10,14-16,34H,2-3,6-8,11-13H2,1H3,(H2,27,35)/t14-,15+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... |
J Med Chem 63: 8584-8607 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00988
BindingDB Entry DOI: 10.7270/Q2DZ0CW6 |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538566

(CHEMBL4632475)Show SMILES Cc1cc(Cl)cc(c1OC1CCNCC1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12 Show InChI InChI=1S/C24H24ClN3O3S/c1-14-10-15(25)11-19(23(14)31-16-4-7-26-8-5-16)18-6-9-27-20-12-17(32-24(18)20)13-28-21(29)2-3-22(28)30/h6,9-12,16,26H,2-5,7-8,13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591079
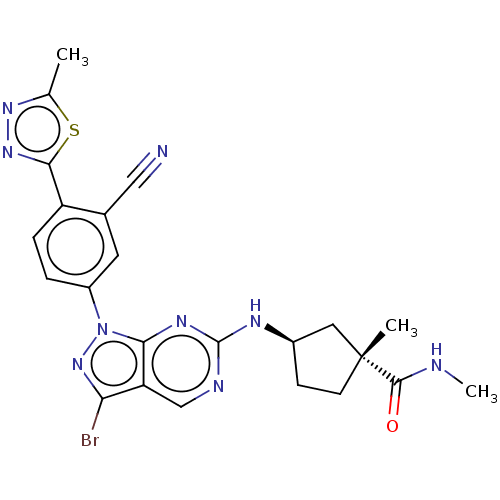
(CHEMBL5196751)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(c3)C#N)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591075

(CHEMBL5200118)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(CCS(C)(=O)=O)c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50545782
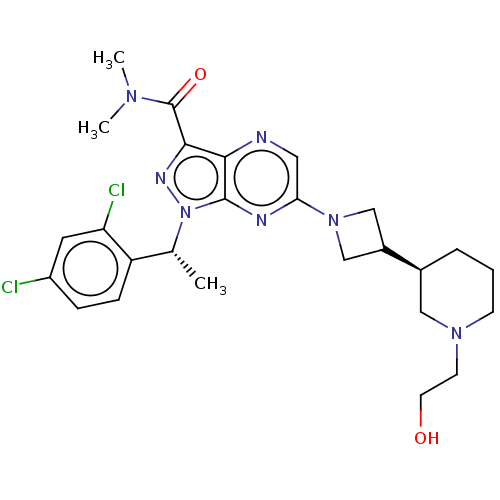
(CHEMBL4637695)Show SMILES C[C@H](c1ccc(Cl)cc1Cl)n1nc(C(=O)N(C)C)c2ncc(nc12)N1CC(C1)[C@H]1CCCN(CCO)C1 |r| Show InChI InChI=1S/C26H33Cl2N7O2/c1-16(20-7-6-19(27)11-21(20)28)35-25-23(24(31-35)26(37)32(2)3)29-12-22(30-25)34-14-18(15-34)17-5-4-8-33(13-17)9-10-36/h6-7,11-12,16-18,36H,4-5,8-10,13-15H2,1-3H3/t16-,17+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... |
J Med Chem 63: 8584-8607 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00988
BindingDB Entry DOI: 10.7270/Q2DZ0CW6 |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538556

(CHEMBL4646914)Show SMILES OCC(O)(CCl)c1cc2nccc(-c3cc(Cl)cc4C[C@@H](Oc34)C(=O)N3CCNCC3)c2s1 |r| Show InChI InChI=1S/C23H23Cl2N3O4S/c24-11-23(31,12-29)19-10-17-21(33-19)15(1-2-27-17)16-9-14(25)7-13-8-18(32-20(13)16)22(30)28-5-3-26-4-6-28/h1-2,7,9-10,18,26,29,31H,3-6,8,11-12H2/t18-,23?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591081
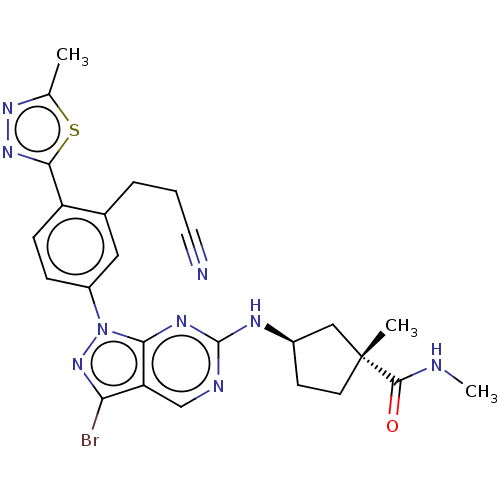
(CHEMBL5193210)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(CCC#N)c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591044
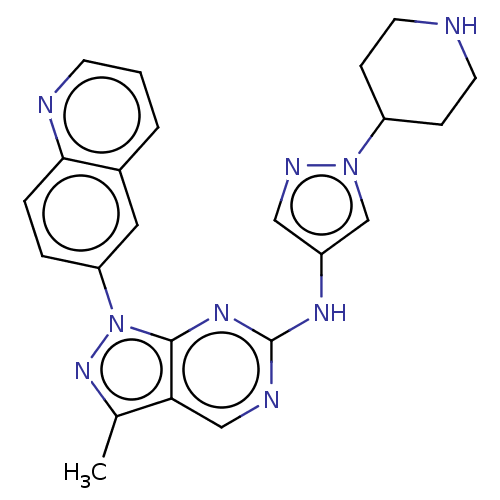
(CHEMBL5202445)Show SMILES Cc1nn(-c2ccc3ncccc3c2)c2nc(Nc3cnn(c3)C3CCNCC3)ncc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50538561
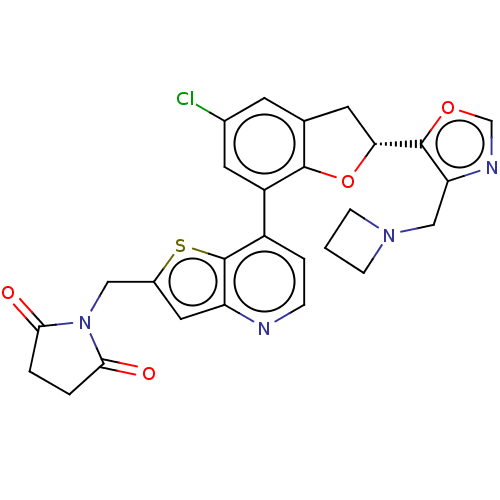
(CHEMBL4642308)Show SMILES Clc1cc2C[C@@H](Oc2c(c1)-c1ccnc2cc(CN3C(=O)CCC3=O)sc12)c1ocnc1CN1CCC1 |r| Show InChI InChI=1S/C27H23ClN4O4S/c28-16-8-15-9-22(26-21(30-14-35-26)13-31-6-1-7-31)36-25(15)19(10-16)18-4-5-29-20-11-17(37-27(18)20)12-32-23(33)2-3-24(32)34/h4-5,8,10-11,14,22H,1-3,6-7,9,12-13H2/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... |
J Med Chem 63: 5398-5420 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00245
BindingDB Entry DOI: 10.7270/Q2XK8K2T |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591077
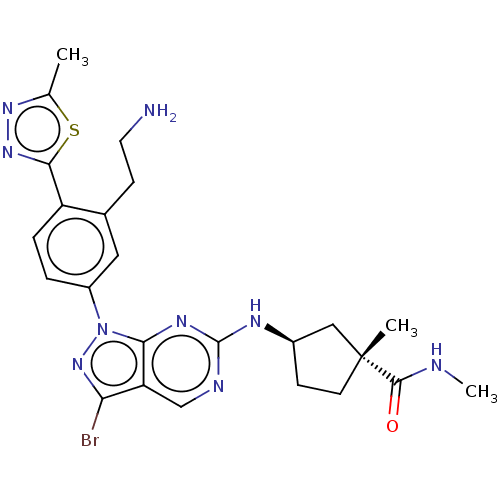
(CHEMBL5201930)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(CCN)c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591065
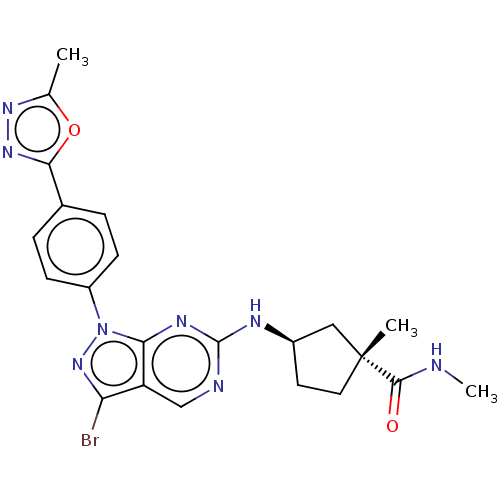
(CHEMBL5170361)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(cc3)-c3nnc(C)o3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591066

(CHEMBL5190023)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(cc3)-c3nnc(C)s3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591065

(CHEMBL5170361)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(cc3)-c3nnc(C)o3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591072
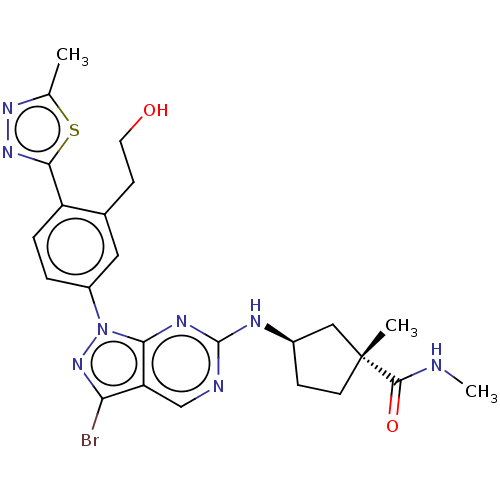
(CHEMBL5172690)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc(-c4nnc(C)s4)c(CCO)c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591064
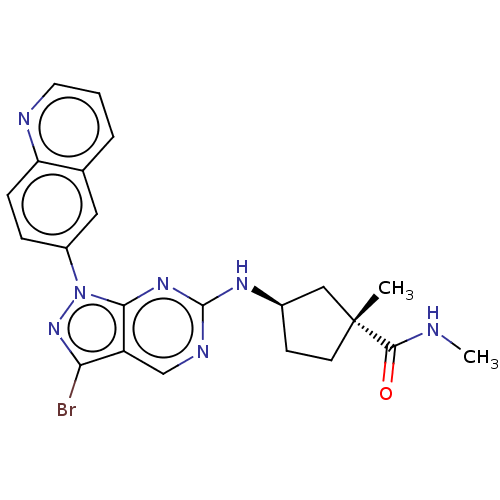
(CHEMBL5199369)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc4ncccc4c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50545769
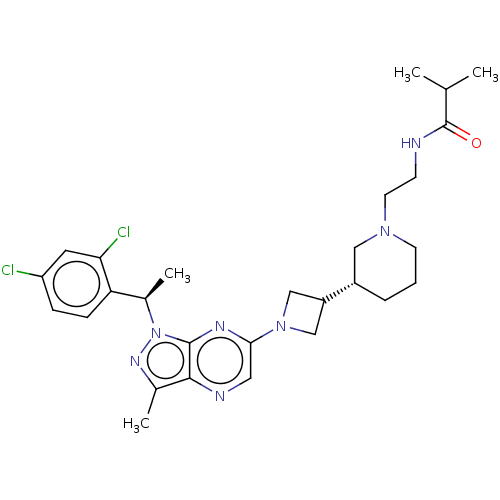
(CHEMBL4645325)Show SMILES CC(C)C(=O)NCCN1CCC[C@@H](C1)C1CN(C1)c1cnc2c(C)nn([C@H](C)c3ccc(Cl)cc3Cl)c2n1 |r| Show InChI InChI=1S/C28H37Cl2N7O/c1-17(2)28(38)31-9-11-35-10-5-6-20(14-35)21-15-36(16-21)25-13-32-26-18(3)34-37(27(26)33-25)19(4)23-8-7-22(29)12-24(23)30/h7-8,12-13,17,19-21H,5-6,9-11,14-16H2,1-4H3,(H,31,38)/t19-,20+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... |
J Med Chem 63: 8584-8607 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00988
BindingDB Entry DOI: 10.7270/Q2DZ0CW6 |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591054

(CHEMBL5179922)Show SMILES COCCNC(=O)[C@@H]1CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc4ncccc4c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591063
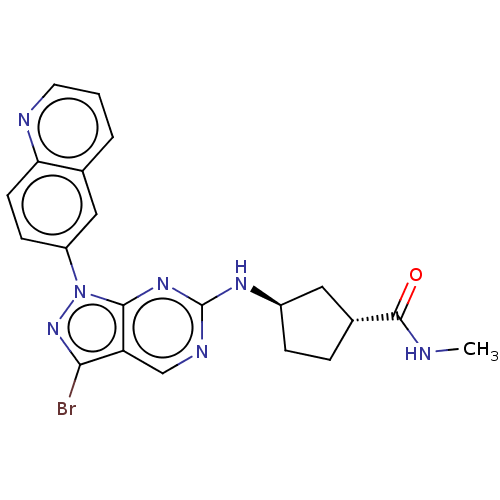
(CHEMBL5180017)Show SMILES CNC(=O)[C@@H]1CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc4ncccc4c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591059
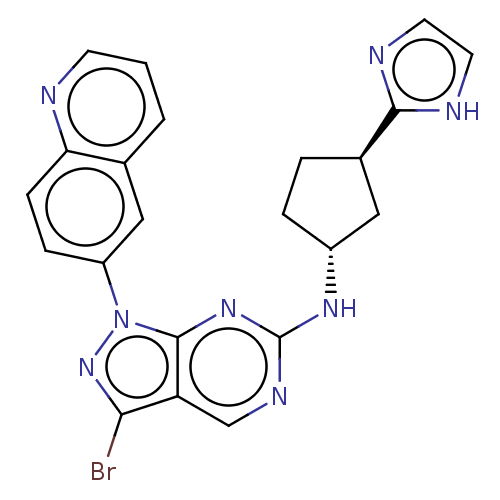
(CHEMBL5195519)Show SMILES Brc1nn(-c2ccc3ncccc3c2)c2nc(N[C@@H]3CC[C@H](C3)c3ncc[nH]3)ncc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50545771
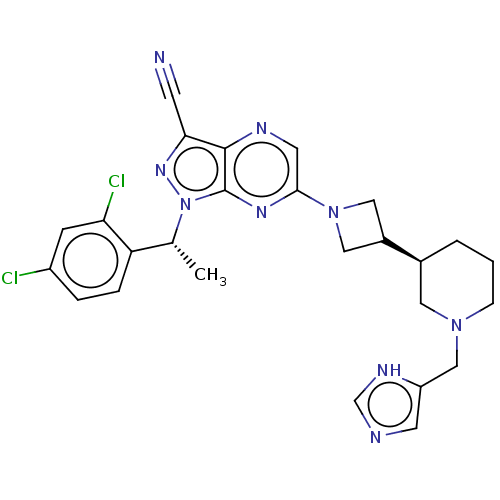
(CHEMBL4639600)Show SMILES C[C@H](c1ccc(Cl)cc1Cl)n1nc(C#N)c2ncc(nc12)N1CC(C1)[C@H]1CCCN(Cc2cnc[nH]2)C1 |r| Show InChI InChI=1S/C26H27Cl2N9/c1-16(21-5-4-19(27)7-22(21)28)37-26-25(23(8-29)34-37)31-10-24(33-26)36-12-18(13-36)17-3-2-6-35(11-17)14-20-9-30-15-32-20/h4-5,7,9-10,15-18H,2-3,6,11-14H2,1H3,(H,30,32)/t16-,17+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... |
J Med Chem 63: 8584-8607 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00988
BindingDB Entry DOI: 10.7270/Q2DZ0CW6 |
More data for this
Ligand-Target Pair | |
eIF-2-alpha kinase GCN2
(Homo sapiens (Human)) | BDBM50591064

(CHEMBL5199369)Show SMILES CNC(=O)[C@]1(C)CC[C@H](C1)Nc1ncc2c(Br)nn(-c3ccc4ncccc4c3)c2n1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00736
BindingDB Entry DOI: 10.7270/Q27H1PJS |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50545778

(CHEMBL4637143)Show SMILES C[C@H](c1ccc(Cl)cc1Cl)n1nc(C#N)c2ncc(nc12)N1CC(C1)[C@H]1CCCN(CCO)C1 |r| Show InChI InChI=1S/C24H27Cl2N7O/c1-15(19-5-4-18(25)9-20(19)26)33-24-23(21(10-27)30-33)28-11-22(29-24)32-13-17(14-32)16-3-2-6-31(12-16)7-8-34/h4-5,9,11,15-17,34H,2-3,6-8,12-14H2,1H3/t15-,16+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... |
J Med Chem 63: 8584-8607 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00988
BindingDB Entry DOI: 10.7270/Q2DZ0CW6 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50545762
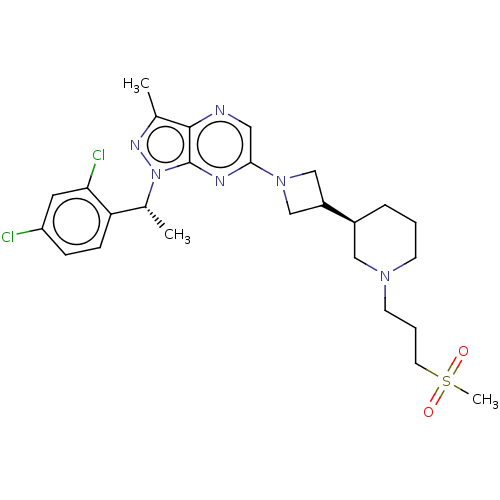
(CHEMBL4647117)Show SMILES C[C@H](c1ccc(Cl)cc1Cl)n1nc(C)c2ncc(nc12)N1CC(C1)[C@H]1CCCN(CCCS(C)(=O)=O)C1 |r| Show InChI InChI=1S/C26H34Cl2N6O2S/c1-17-25-26(34(31-17)18(2)22-8-7-21(27)12-23(22)28)30-24(13-29-25)33-15-20(16-33)19-6-4-9-32(14-19)10-5-11-37(3,35)36/h7-8,12-13,18-20H,4-6,9-11,14-16H2,1-3H3/t18-,19+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... |
J Med Chem 63: 8584-8607 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00988
BindingDB Entry DOI: 10.7270/Q2DZ0CW6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data