

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM400979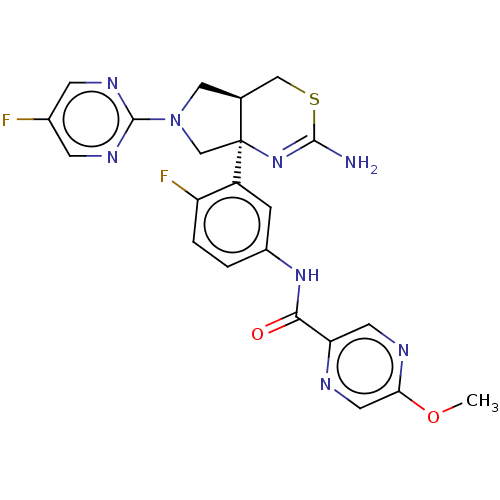 (US9999624, Compound 4) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM150693 (US8987254, 8 | US9999624, 9) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM400643 (N-[3-[(4aR,7aS)-2-Amino-6-(5-fluoropyrimidin-2-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.358 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20× in KH2PO4 buffer. Ten μL of each dilution ... | Bioorg Med Chem 17: 7301-12 (2009) BindingDB Entry DOI: 10.7270/Q27H1MZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150692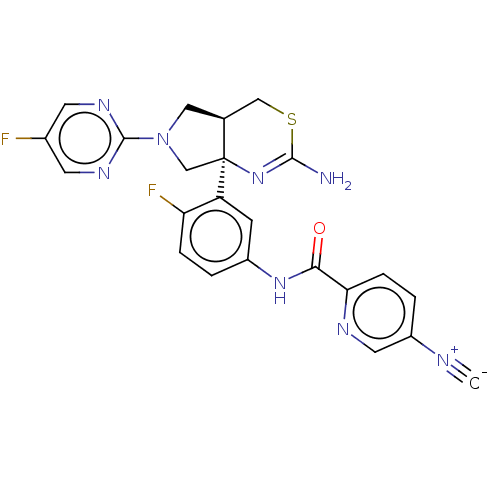 (US8987254, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.358 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US8987254 (2015) BindingDB Entry DOI: 10.7270/Q2FX786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM150688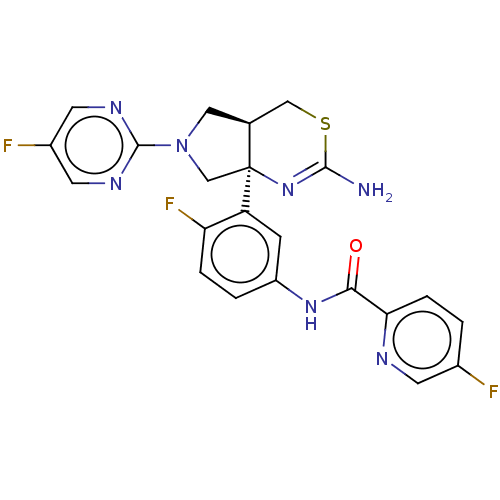 (US8987254, 3 | US9999624, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.388 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150690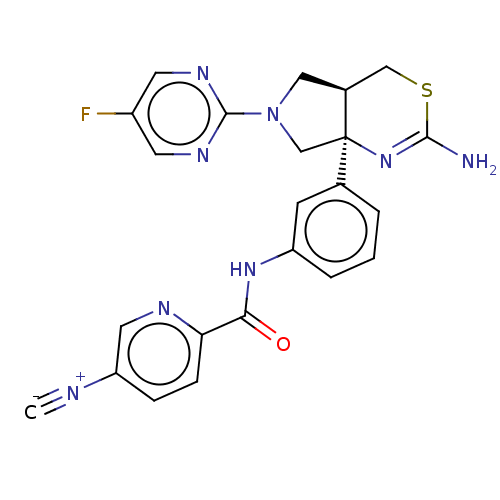 (US8987254, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US8987254 (2015) BindingDB Entry DOI: 10.7270/Q2FX786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM400607 (US9999624, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20× in KH2PO4 buffer. Ten μL of each dilution ... | Bioorg Med Chem 17: 7301-12 (2009) BindingDB Entry DOI: 10.7270/Q27H1MZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM150688 (US8987254, 3 | US9999624, 3) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.481 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150687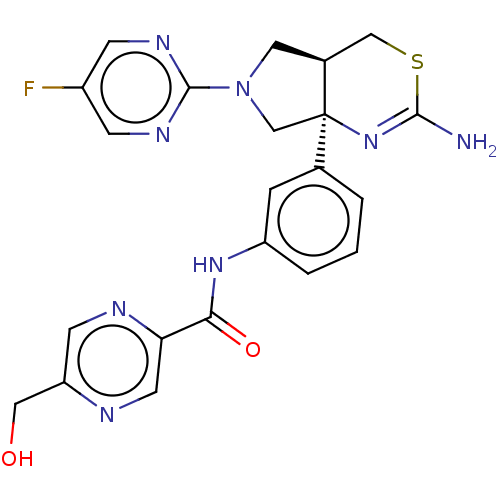 (US8987254, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.482 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US8987254 (2015) BindingDB Entry DOI: 10.7270/Q2FX786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM400331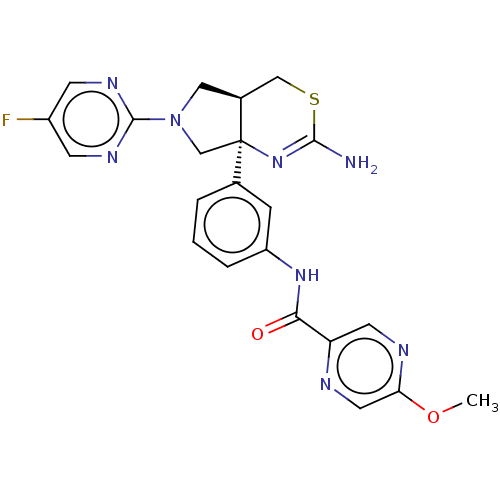 (US9999624, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.482 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20× in KH2PO4 buffer. Ten μL of each dilution ... | Bioorg Med Chem 17: 7301-12 (2009) BindingDB Entry DOI: 10.7270/Q27H1MZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150688 (US8987254, 3 | US9999624, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.554 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US8987254 (2015) BindingDB Entry DOI: 10.7270/Q2FX786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM150693 (US8987254, 8 | US9999624, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.555 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150689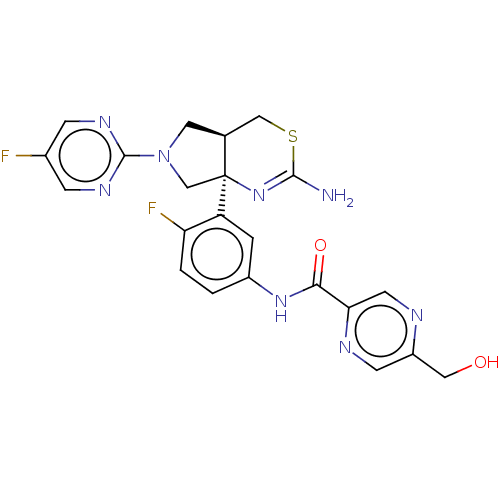 (US8987254, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.569 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US8987254 (2015) BindingDB Entry DOI: 10.7270/Q2FX786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150688 (US8987254, 3 | US9999624, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20× in KH2PO4 buffer. Ten μL of each dilution ... | Bioorg Med Chem 17: 7301-12 (2009) BindingDB Entry DOI: 10.7270/Q27H1MZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150688 (US8987254, 3 | US9999624, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150686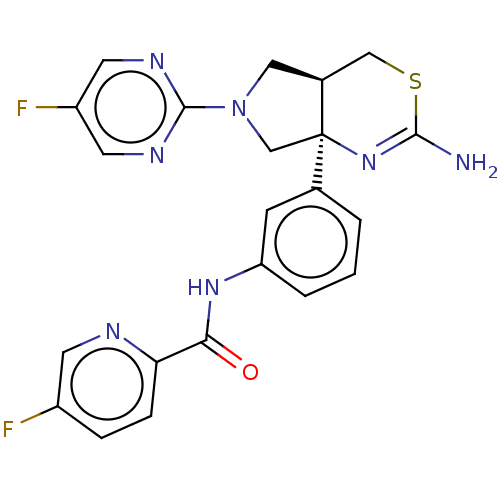 (US8987254, 1 | US9999624, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20× in KH2PO4 buffer. Ten μL of each dilution ... | Bioorg Med Chem 17: 7301-12 (2009) BindingDB Entry DOI: 10.7270/Q27H1MZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150686 (US8987254, 1 | US9999624, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US8987254 (2015) BindingDB Entry DOI: 10.7270/Q2FX786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM400979 (US9999624, Compound 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 0.615 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20× in KH2PO4 buffer. Ten μL of each dilution ... | Bioorg Med Chem 17: 7301-12 (2009) BindingDB Entry DOI: 10.7270/Q27H1MZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM400979 (US9999624, Compound 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.615 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150693 (US8987254, 8 | US9999624, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US8987254 (2015) BindingDB Entry DOI: 10.7270/Q2FX786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150691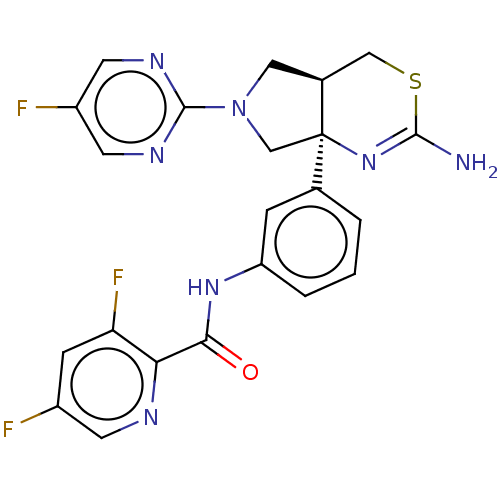 (US8987254, 6 | US9999624, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.739 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US8987254 (2015) BindingDB Entry DOI: 10.7270/Q2FX786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150691 (US8987254, 6 | US9999624, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.739 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20× in KH2PO4 buffer. Ten μL of each dilution ... | Bioorg Med Chem 17: 7301-12 (2009) BindingDB Entry DOI: 10.7270/Q27H1MZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150693 (US8987254, 8 | US9999624, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20× in KH2PO4 buffer. Ten μL of each dilution ... | Bioorg Med Chem 17: 7301-12 (2009) BindingDB Entry DOI: 10.7270/Q27H1MZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM150693 (US8987254, 8 | US9999624, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM400979 (US9999624, Compound 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012647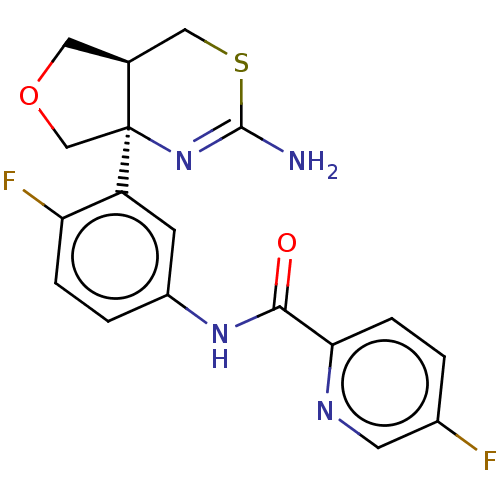 (CHEMBL2396989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50540172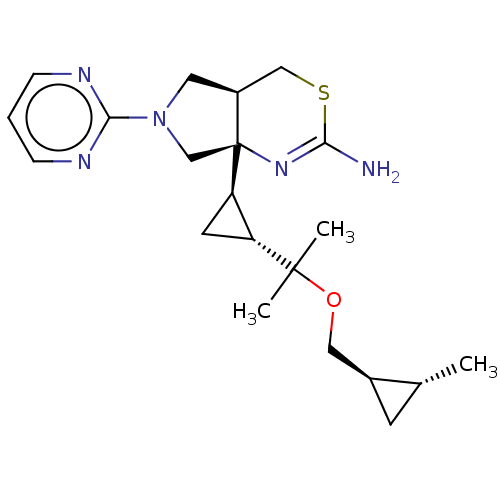 (CHEMBL4637426) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE2 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM158179 (US9029367, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.66 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US9029367 (2015) BindingDB Entry DOI: 10.7270/Q21Z4344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50012647 (CHEMBL2396989) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE2 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540172 (CHEMBL4637426) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540172 (CHEMBL4637426) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Mus musculus (Mouse)) | BDBM50012647 (CHEMBL2396989) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM158178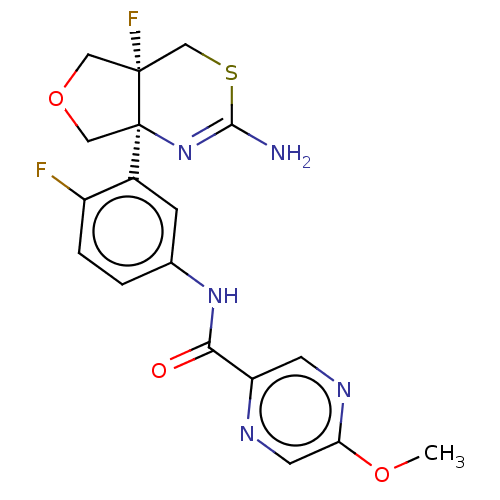 (US9029367, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US9029367 (2015) BindingDB Entry DOI: 10.7270/Q21Z4344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM158177 (US9029367, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15.6 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US9029367 (2015) BindingDB Entry DOI: 10.7270/Q21Z4344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540171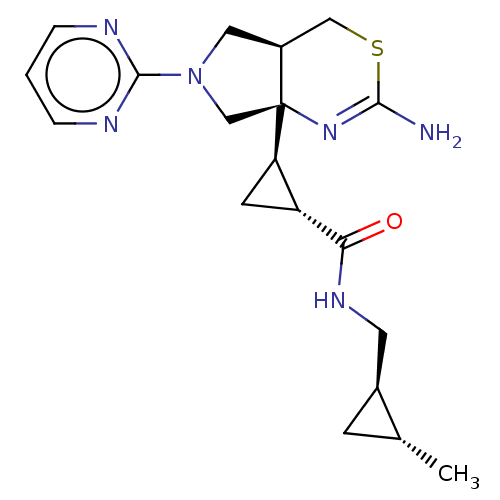 (CHEMBL4643727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) by cell based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012647 (CHEMBL2396989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50540171 (CHEMBL4643727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE2 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM158181 (US9029367, 57) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 25.7 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US9029367 (2015) BindingDB Entry DOI: 10.7270/Q21Z4344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540171 (CHEMBL4643727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540171 (CHEMBL4643727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50540170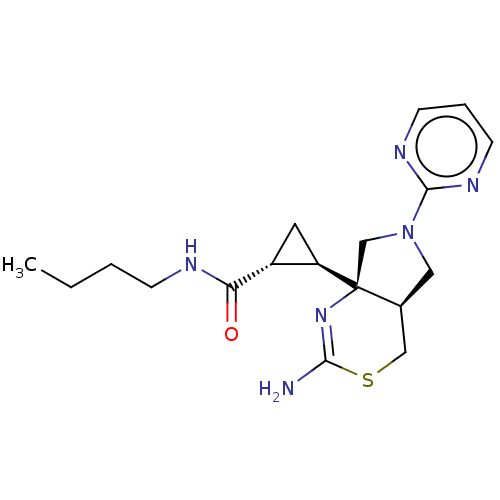 (CHEMBL4636286) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE2 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM158180 (US9029367, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad... | US Patent US9029367 (2015) BindingDB Entry DOI: 10.7270/Q21Z4344 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM126005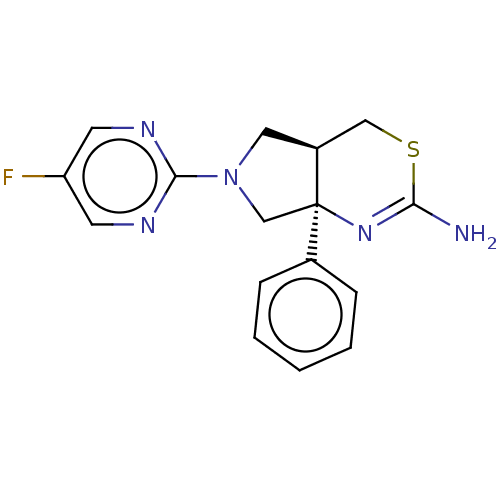 (US8772282, 1 | US8772282, 203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64.4 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten μL of each dilution ... | US Patent US8772282 (2014) BindingDB Entry DOI: 10.7270/Q2JH3JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50540170 (CHEMBL4636286) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) expressed in HEK293 cells using mcaFRET peptide as substrate after 20 hrs by FRET assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115194 BindingDB Entry DOI: 10.7270/Q2B56P8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-460] (Homo sapiens (Human)) | BDBM224809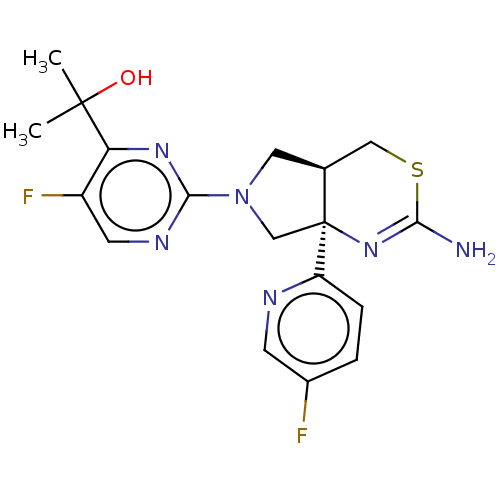 (US9328124, 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 73.4 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Ten μL of each dilution is added to each well on row A to H of a corresponding low protein binding black plate containing the reaction mixture (25... | US Patent US9328124 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM126007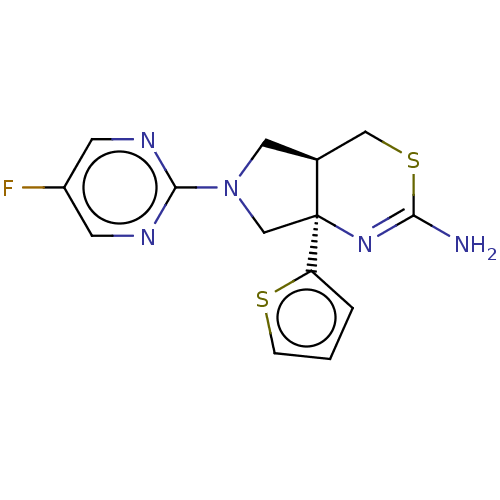 (US8772282, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 77.3 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten μL of each dilution ... | US Patent US8772282 (2014) BindingDB Entry DOI: 10.7270/Q2JH3JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50581133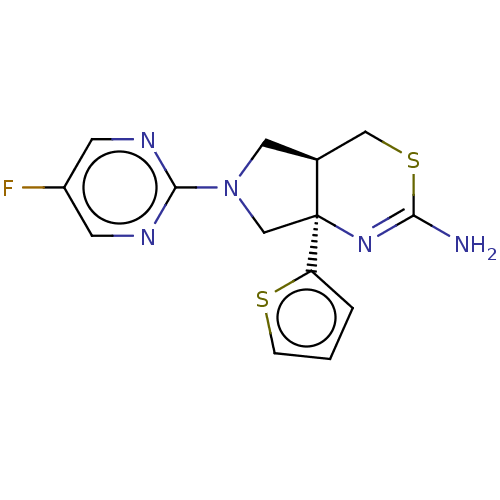 (CHEMBL5093835) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM126012 (US8772282, 32) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 91.2 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten μL of each dilution ... | US Patent US8772282 (2014) BindingDB Entry DOI: 10.7270/Q2JH3JVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-460] (Homo sapiens (Human)) | BDBM224810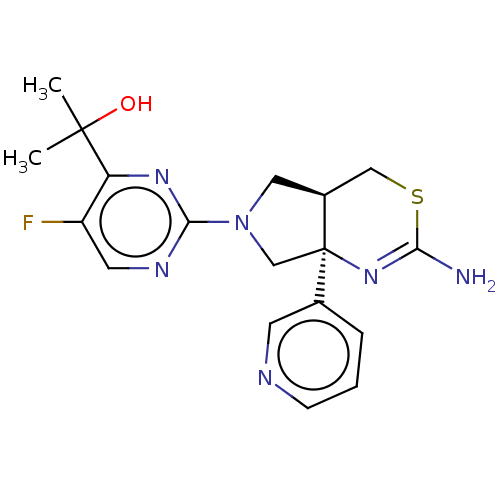 (US9328124, 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 93.5 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Ten μL of each dilution is added to each well on row A to H of a corresponding low protein binding black plate containing the reaction mixture (25... | US Patent US9328124 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-460] (Homo sapiens (Human)) | BDBM224808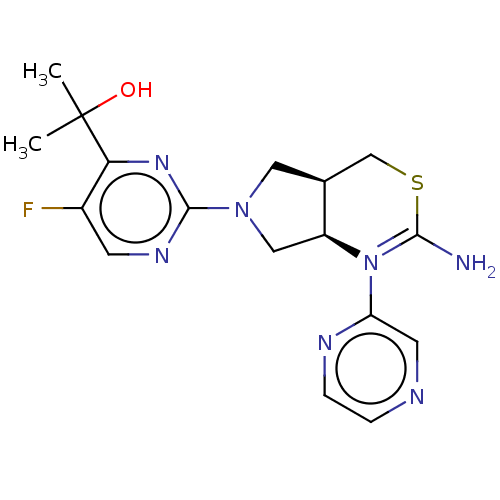 (US9328124, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 105 | n/a | n/a | n/a | n/a | 4.6 | 25 |
Eli Lilly and Company US Patent | Assay Description Ten μL of each dilution is added to each well on row A to H of a corresponding low protein binding black plate containing the reaction mixture (25... | US Patent US9328124 (2016) BindingDB Entry DOI: 10.7270/Q2KH0M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 128 total ) | Next | Last >> |