

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513990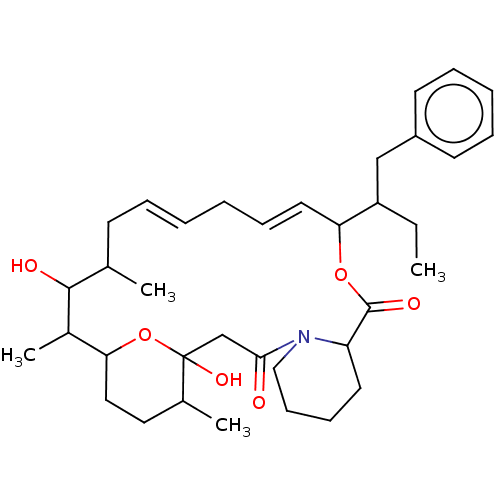 (US11059830, Compound 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding of compounds of the invention to FKBP12 can be determined using the following protocol.General ProtocolThis protocol utilizes Perkin Elme... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513996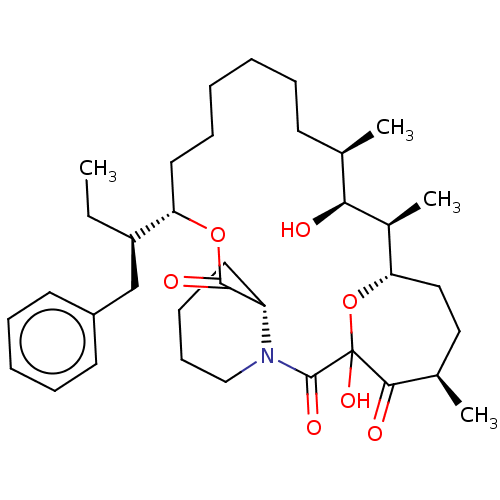 ((2S)-1-((4R,7S)-7-((2R,3S,4R,11 S,12R)-12-benzyl-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 21.5 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This protocol utilizes Surface Plasmon Resonance (SPR) as a method to determine kinetics (KD, Ka, Kd) for the binding of compound (analyte) to immobi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513993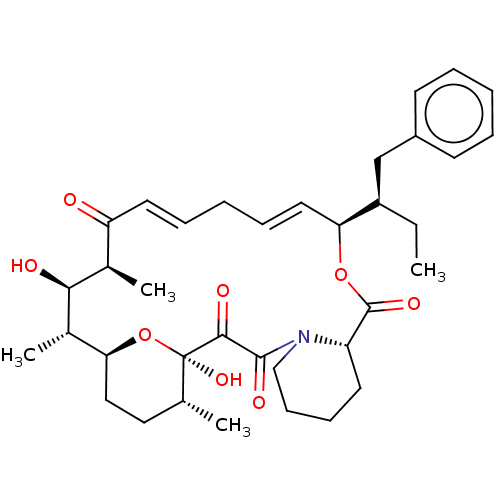 (US11059830, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding of compounds of the invention to FKBP12 can be determined using the following protocol.General ProtocolThis protocol utilizes Perkin Elme... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513994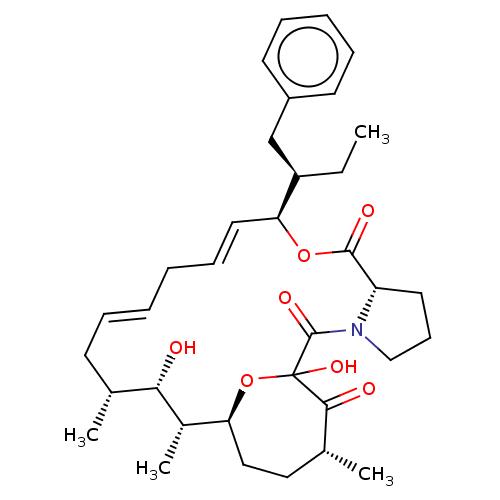 (US11059830, Compound 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding of compounds of the invention to FKBP12 can be determined using the following protocol.General ProtocolThis protocol utilizes Perkin Elme... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513995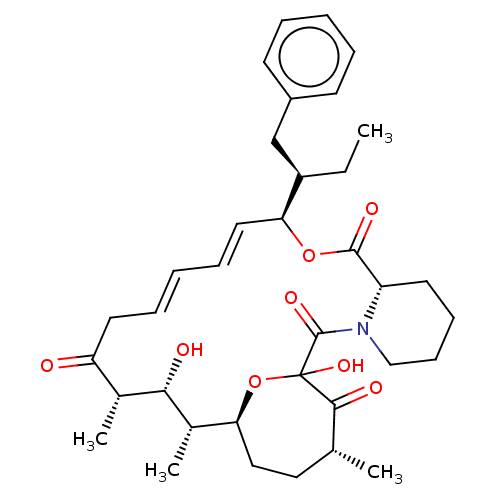 (US11059830, Compound 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 18.8 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding of compounds of the invention to FKBP12 can be determined using the following protocol.General ProtocolThis protocol utilizes Perkin Elme... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513996 ((2S)-1-((4R,7S)-7-((2R,3S,4R,11 S,12R)-12-benzyl-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding of compounds of the invention to FKBP12 can be determined using the following protocol.General ProtocolThis protocol utilizes Perkin Elme... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513997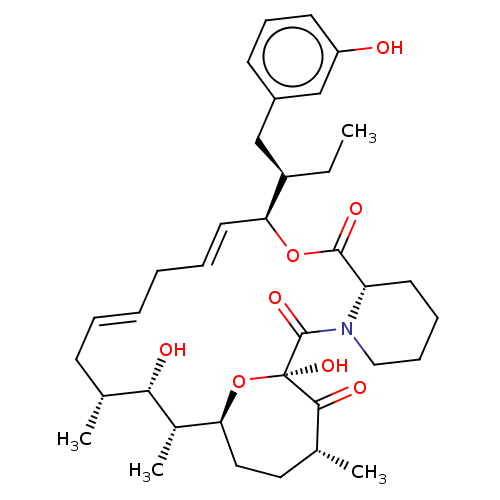 (US11059830, Compound 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding of compounds of the invention to FKBP12 can be determined using the following protocol.General ProtocolThis protocol utilizes Perkin Elme... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513998 (US11059830, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding of compounds of the invention to FKBP12 can be determined using the following protocol.General ProtocolThis protocol utilizes Perkin Elme... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513999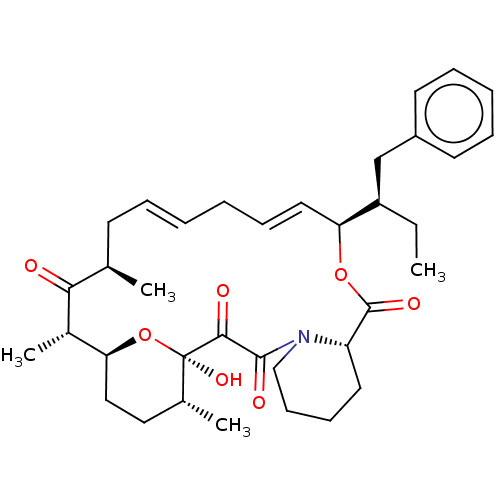 (US11059830, Compound 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 785 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding of compounds of the invention to FKBP12 can be determined using the following protocol.General ProtocolThis protocol utilizes Perkin Elme... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM514000 ((S)-1-(2-((2R,3R,6S)-6-((2R,3R,4S,6E,9E,11R,12R)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding of compounds of the invention to FKBP12 can be determined using the following protocol.General ProtocolThis protocol utilizes Perkin Elme... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513990 (US11059830, Compound 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 71.5 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This protocol utilizes Surface Plasmon Resonance (SPR) as a method to determine kinetics (KD, Ka, Kd) for the binding of compound (analyte) to immobi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513992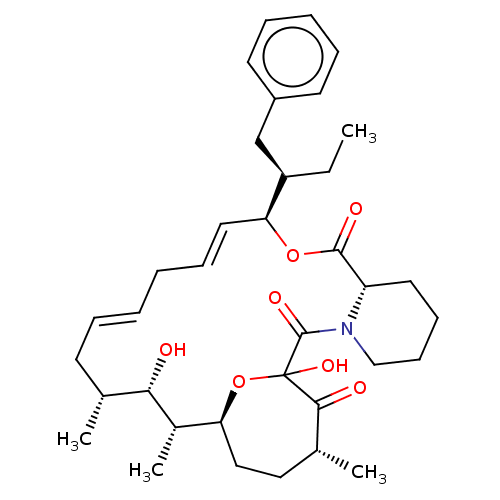 (US11059830, Compound 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This protocol utilizes Surface Plasmon Resonance (SPR) as a method to determine kinetics (KD, Ka, Kd) for the binding of compound (analyte) to immobi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513993 (US11059830, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This protocol utilizes Surface Plasmon Resonance (SPR) as a method to determine kinetics (KD, Ka, Kd) for the binding of compound (analyte) to immobi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513994 (US11059830, Compound 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 23.1 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This protocol utilizes Surface Plasmon Resonance (SPR) as a method to determine kinetics (KD, Ka, Kd) for the binding of compound (analyte) to immobi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513995 (US11059830, Compound 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This protocol utilizes Surface Plasmon Resonance (SPR) as a method to determine kinetics (KD, Ka, Kd) for the binding of compound (analyte) to immobi... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM513992 (US11059830, Compound 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding of compounds of the invention to FKBP12 can be determined using the following protocol.General ProtocolThis protocol utilizes Perkin Elme... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JS9TKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||