

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50003524 (CHEMBL3216197 | [4-(2,2-Diphenyl-propionyl)-pipera...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach& C£a.S.A. Curated by ChEMBL | Assay Description Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%. | J Med Chem 35: 4118-34 (1992) BindingDB Entry DOI: 10.7270/Q228087F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131579 (4-[4-Chloro-5-(4-ethoxy-phenyl)-imidazol-1-yl]-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (COX-2) in 143982 cells | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131567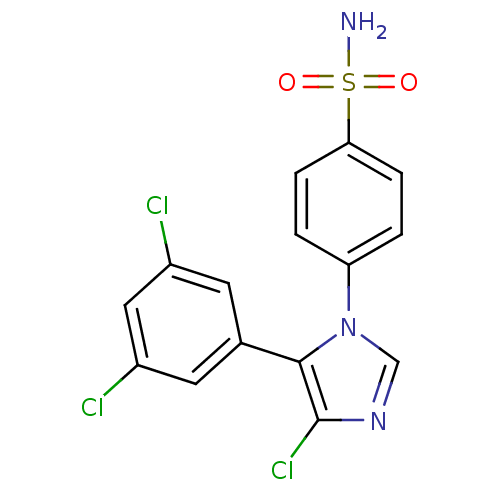 (4-[4-Chloro-5-(3,5-dichloro-phenyl)-imidazol-1-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 1 (COX-1) in U-937 cells | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50005224 (2-({Acetyl-[5-(16-methoxy-hexadecyloxy)-tetrahydro...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description In vitro inhibition of PAF induced aggregation of rabbit platelets. | J Med Chem 35: 676-83 (1992) BindingDB Entry DOI: 10.7270/Q298860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50003499 (CHEMBL338940 | [4-(3-Hydroxy-3,3-diphenyl-propiony...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach& C£a.S.A. Curated by ChEMBL | Assay Description Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%. | J Med Chem 35: 4118-34 (1992) BindingDB Entry DOI: 10.7270/Q228087F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131569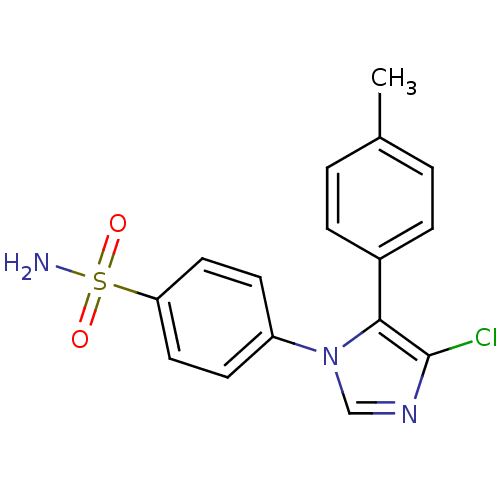 (4-(4-Chloro-5-p-tolyl-imidazol-1-yl)-benzenesulfon...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131585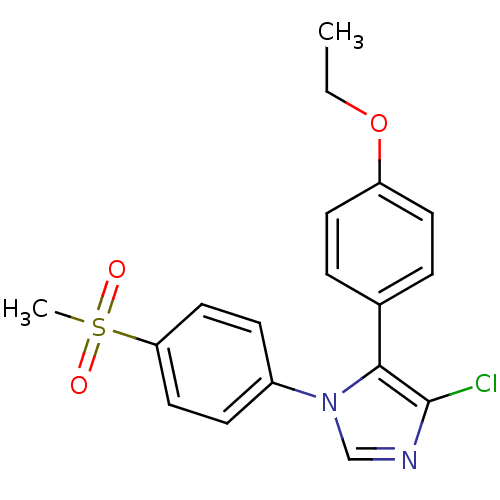 (4-Chloro-5-(4-ethoxy-phenyl)-1-(4-methanesulfonyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131622 (4-Chloro-5-(3-fluoro-4-methoxy-phenyl)-1-(4-methan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50005227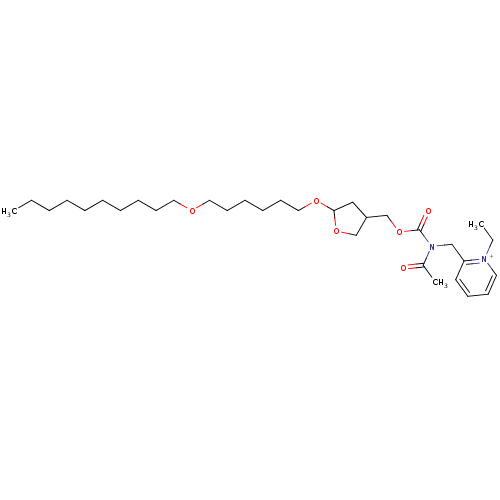 (2-({Acetyl-[5-(6-decyloxy-hexyloxy)-tetrahydro-fur...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description In vitro inhibition of PAF induced aggregation of rabbit platelets. | J Med Chem 35: 676-83 (1992) BindingDB Entry DOI: 10.7270/Q298860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50011053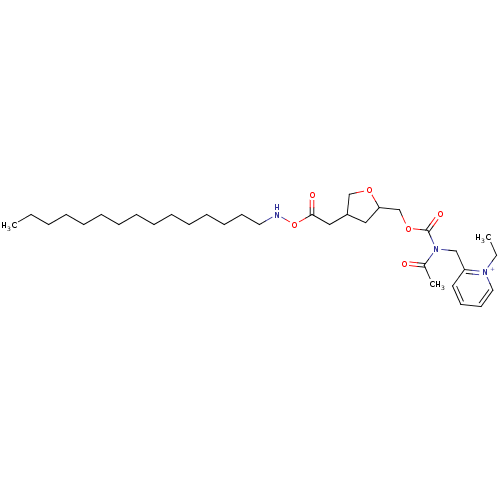 (CHEMBL125576 | Tetrahydrofuran derivative) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description Inhibitory activity expressed as the concentration required to inhibit platelet-activating factor (PAF) induced maximum aggregation by 50% | J Med Chem 34: 373-86 (1991) BindingDB Entry DOI: 10.7270/Q2DF6Q51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131646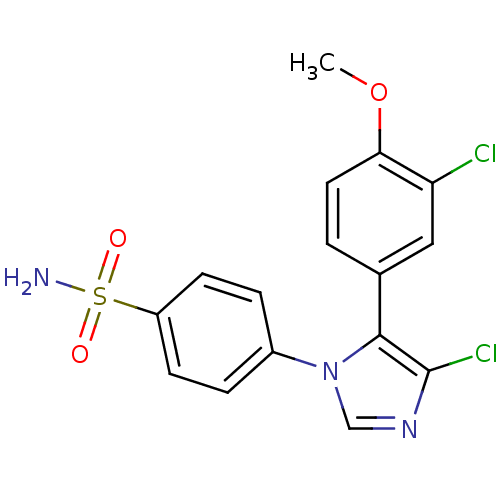 (4-[4-Chloro-5-(3-chloro-4-methoxy-phenyl)-imidazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131593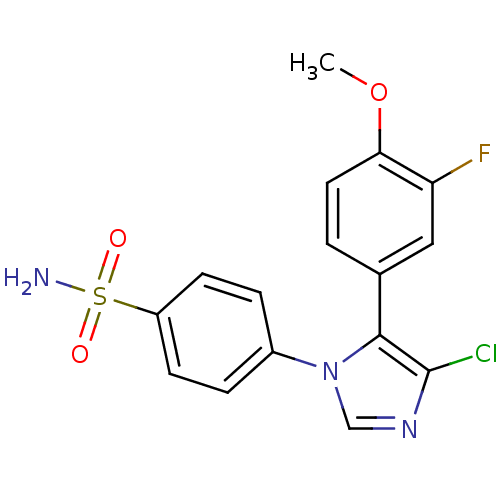 (4-[4-Chloro-5-(3-fluoro-4-methoxy-phenyl)-imidazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131604 (5-Benzo[1,3]dioxol-5-yl-4-chloro-1-(4-methanesulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131602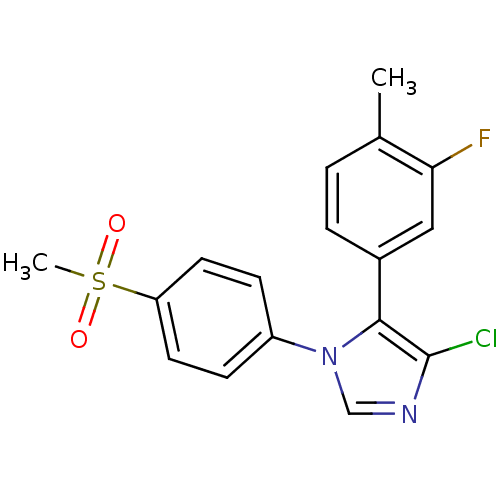 (4-Chloro-5-(3-fluoro-4-methyl-phenyl)-1-(4-methane...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131608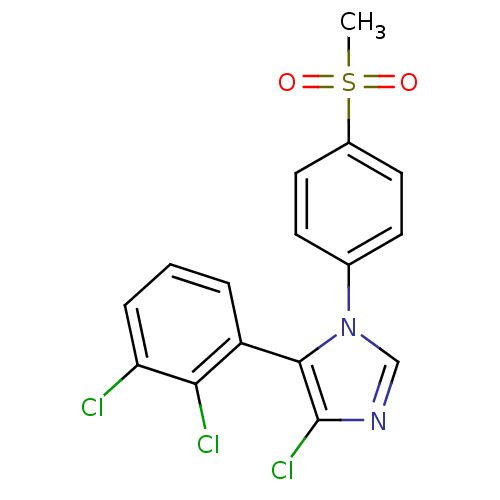 (4-Chloro-5-(2,3-dichloro-phenyl)-1-(4-methanesulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50005225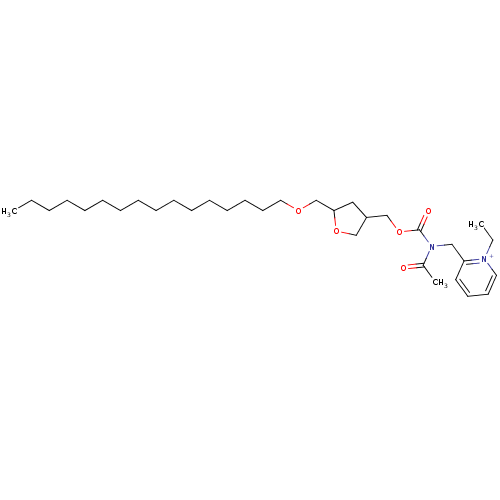 (2-{[Acetyl-(5-hexadecyloxymethyl-tetrahydro-furan-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description Inhibitory activity expressed as the concentration required to inhibit platelet-activating factor (PAF) induced maximum aggregation by 50% | J Med Chem 34: 373-86 (1991) BindingDB Entry DOI: 10.7270/Q2DF6Q51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131591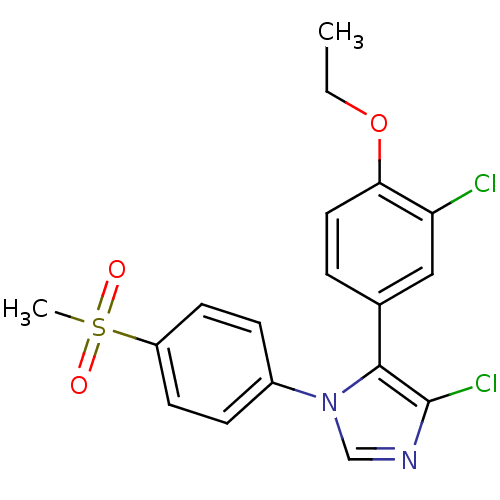 (4-Chloro-5-(3-chloro-4-ethoxy-phenyl)-1-(4-methane...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131614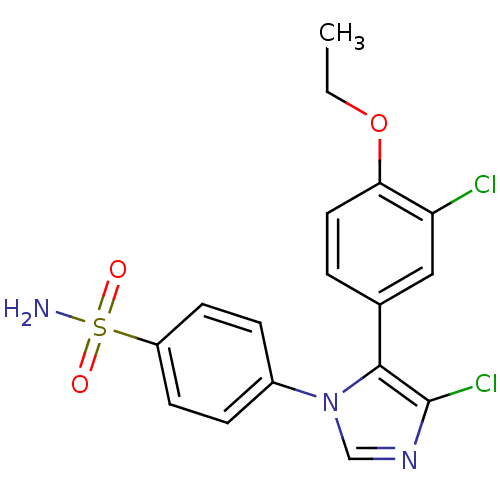 (4-[4-Chloro-5-(3-chloro-4-ethoxy-phenyl)-imidazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131650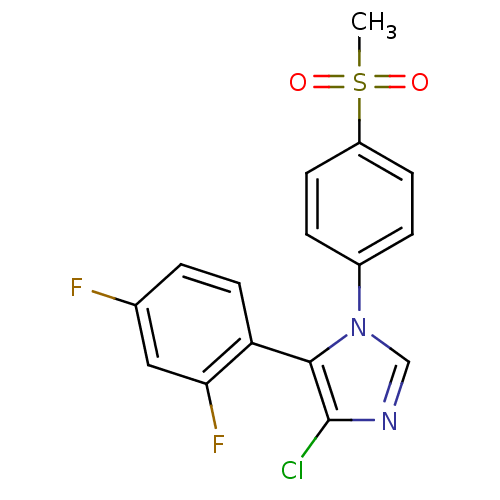 (4-Chloro-5-(2,4-difluoro-phenyl)-1-(4-methanesulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131611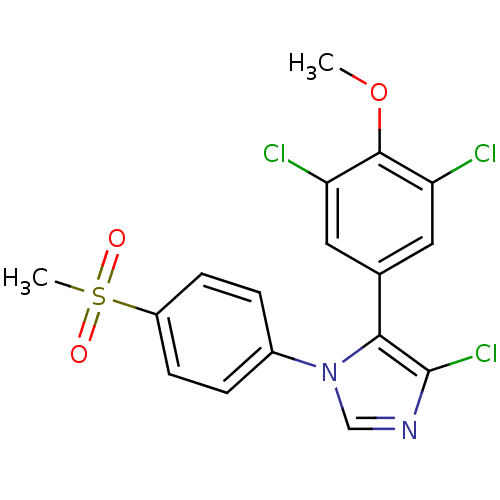 (4-Chloro-5-(3,5-dichloro-4-methoxy-phenyl)-1-(4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50003525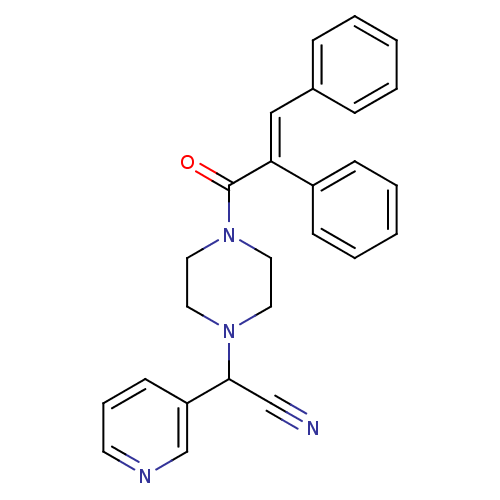 (CHEMBL133742 | [4-(2,3-Diphenyl-acryloyl)-piperazi...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach& C£a.S.A. Curated by ChEMBL | Assay Description Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%. | J Med Chem 35: 4118-34 (1992) BindingDB Entry DOI: 10.7270/Q228087F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50011106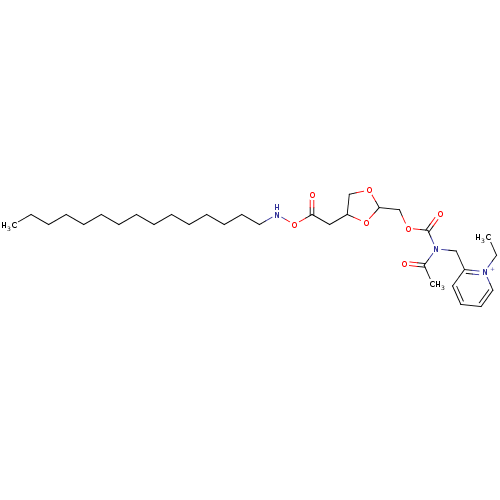 (2-{[Acetyl-(4-octadecyloxymethyl-[1,3]dioxolan-2-y...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description Inhibitory activity expressed as the concentration required to inhibit platelet-activating factor (PAF) induced maximum aggregation by 50% | J Med Chem 34: 373-86 (1991) BindingDB Entry DOI: 10.7270/Q2DF6Q51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131616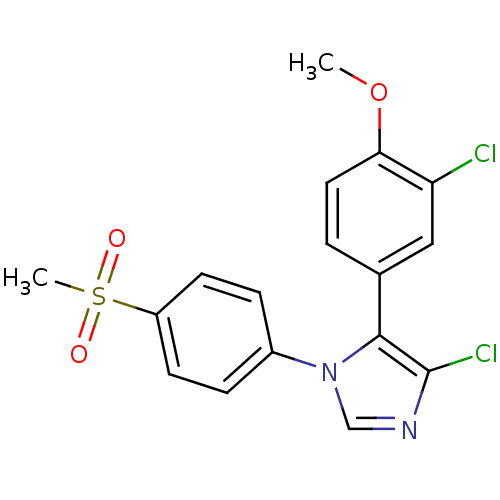 (4-Chloro-5-(3-chloro-4-methoxy-phenyl)-1-(4-methan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50011053 (CHEMBL125576 | Tetrahydrofuran derivative) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description Inhibitory activity expressed as the concentration required to inhibit platelet-activating factor (PAF) induced maximum aggregation by 50% | J Med Chem 34: 373-86 (1991) BindingDB Entry DOI: 10.7270/Q2DF6Q51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131647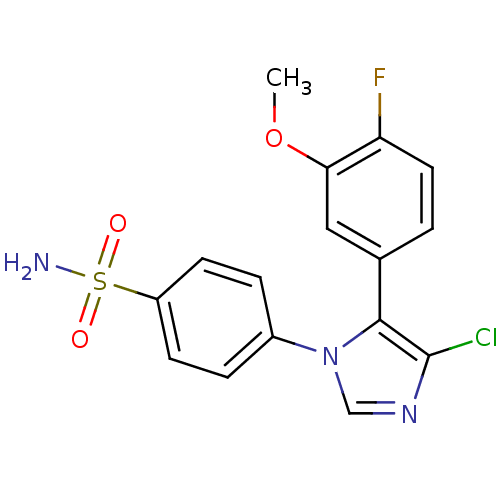 (4-[4-Chloro-5-(4-fluoro-3-methoxy-phenyl)-imidazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50011055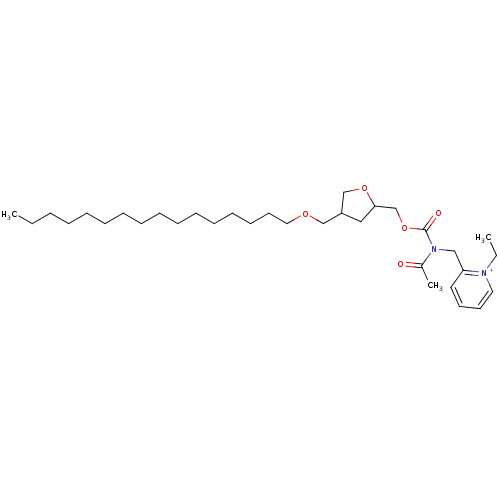 (2-{[Acetyl-(4-hexadecyloxymethyl-tetrahydro-furan-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description Inhibitory activity expressed as the concentration required to inhibit platelet-activating factor (PAF) induced maximum aggregation by 50% | J Med Chem 34: 373-86 (1991) BindingDB Entry DOI: 10.7270/Q2DF6Q51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50005225 (2-{[Acetyl-(5-hexadecyloxymethyl-tetrahydro-furan-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description Inhibitory activity expressed as the concentration required to inhibit platelet-activating factor (PAF) induced maximum aggregation by 50% | J Med Chem 34: 373-86 (1991) BindingDB Entry DOI: 10.7270/Q2DF6Q51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50005225 (2-{[Acetyl-(5-hexadecyloxymethyl-tetrahydro-furan-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description In vitro inhibition of PAF induced aggregation of rabbit platelets. | J Med Chem 35: 676-83 (1992) BindingDB Entry DOI: 10.7270/Q298860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50045990 (CHEMBL102070 | N-(3-{4-[Cyano-(2-methyl-pyridin-3-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibition of PAF-induced platelet aggregation | J Med Chem 36: 2984-97 (1993) BindingDB Entry DOI: 10.7270/Q2ZS2VKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131621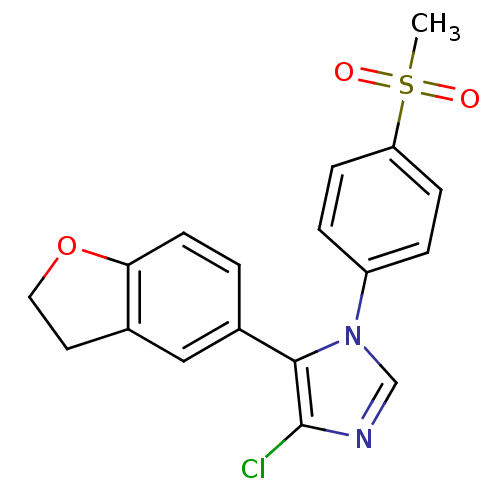 (4-Chloro-5-(2,3-dihydro-benzofuran-5-yl)-1-(4-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131586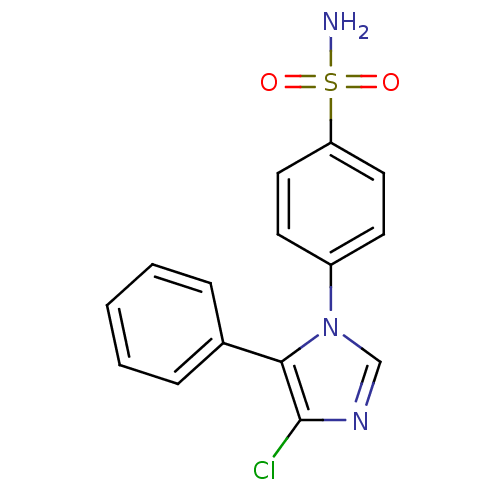 (4-(4-Chloro-5-phenyl-imidazol-1-yl)-benzenesulfona...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50045970 (CHEMBL102918 | N-(3-{4-[Cyano-(2-methyl-pyridin-3-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibition of PAF-induced platelet aggregation | J Med Chem 36: 2984-97 (1993) BindingDB Entry DOI: 10.7270/Q2ZS2VKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50003506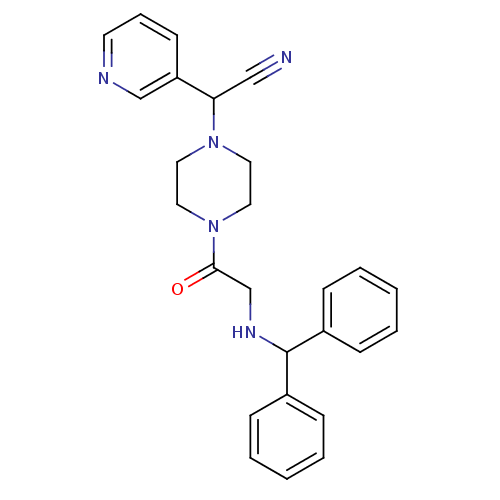 (CHEMBL322184 | CHEMBL545771 | {4-[2-(Benzhydryl-am...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibition of PAF-induced platelet aggregation | J Med Chem 36: 2984-97 (1993) BindingDB Entry DOI: 10.7270/Q2ZS2VKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50003506 (CHEMBL322184 | CHEMBL545771 | {4-[2-(Benzhydryl-am...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach& C£a.S.A. Curated by ChEMBL | Assay Description Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%. | J Med Chem 35: 4118-34 (1992) BindingDB Entry DOI: 10.7270/Q228087F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50046012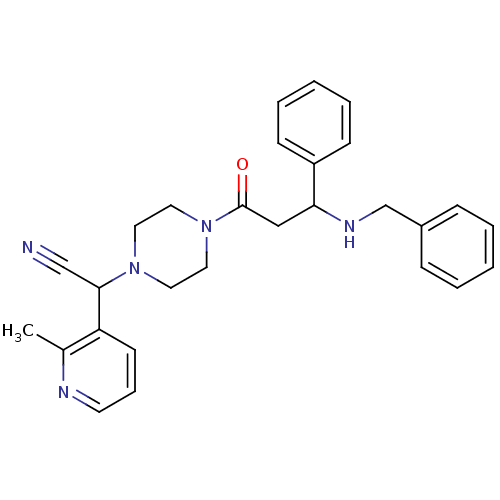 (CHEMBL100497 | [4-(3-Benzylamino-3-phenyl-propiony...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibition of PAF-induced platelet aggregation | J Med Chem 36: 2984-97 (1993) BindingDB Entry DOI: 10.7270/Q2ZS2VKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50005231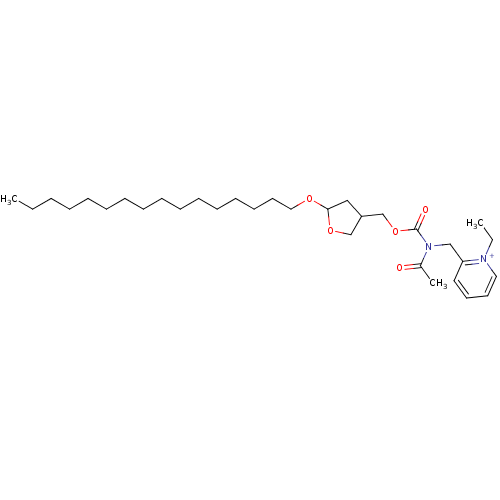 (2-{[Acetyl-(5-hexadecyloxy-tetrahydro-furan-3-ylme...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description In vitro inhibition of PAF induced aggregation of rabbit platelets. | J Med Chem 35: 676-83 (1992) BindingDB Entry DOI: 10.7270/Q298860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50003473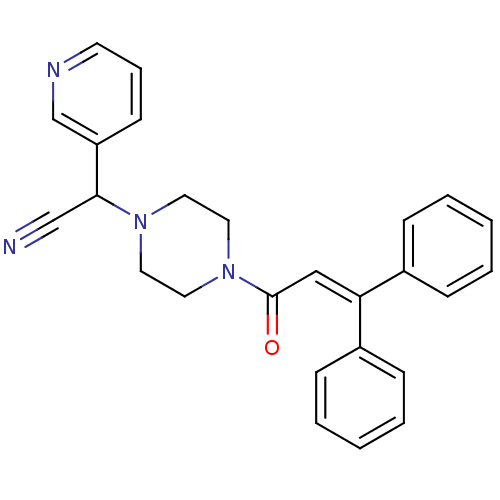 (CHEMBL317193 | [4-(3,3-Diphenyl-acryloyl)-piperazi...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description In vitro inhibition of PAF-induced platelet aggregation in male rabbits | J Med Chem 36: 2984-97 (1993) BindingDB Entry DOI: 10.7270/Q2ZS2VKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50003473 (CHEMBL317193 | [4-(3,3-Diphenyl-acryloyl)-piperazi...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach& C£a.S.A. Curated by ChEMBL | Assay Description Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%. | J Med Chem 35: 4118-34 (1992) BindingDB Entry DOI: 10.7270/Q228087F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50003522 ((4-Diphenylacetyl-piperazin-1-yl)-pyridin-3-yl-ace...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach& C£a.S.A. Curated by ChEMBL | Assay Description Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%. | J Med Chem 35: 4118-34 (1992) BindingDB Entry DOI: 10.7270/Q228087F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131648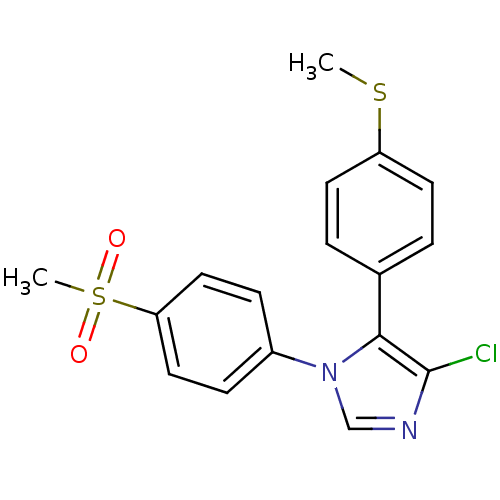 (4-Chloro-1-(4-methanesulfonyl-phenyl)-5-(4-methyls...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50011058 (CHEMBL124534 | Tetrahydrofuran derivative) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description Inhibitory activity expressed as the concentration required to inhibit platelet-activating factor (PAF) induced maximum aggregation by 50% | J Med Chem 34: 373-86 (1991) BindingDB Entry DOI: 10.7270/Q2DF6Q51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50011058 (CHEMBL124534 | Tetrahydrofuran derivative) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description Inhibitory activity expressed as the concentration required to inhibit platelet-activating factor (PAF) induced maximum aggregation by 50% | J Med Chem 34: 373-86 (1991) BindingDB Entry DOI: 10.7270/Q2DF6Q51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50005237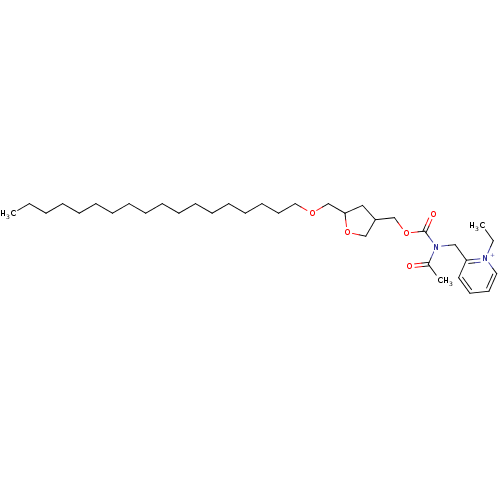 (2-{[Acetyl-(5-octadecyloxymethyl-tetrahydro-furan-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description In vitro inhibition of PAF induced aggregation of rabbit platelets. | J Med Chem 35: 676-83 (1992) BindingDB Entry DOI: 10.7270/Q298860P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50003514 (CHEMBL3216401 | [4-(2-Benzyl-3-phenyl-propionyl)-p...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach& C£a.S.A. Curated by ChEMBL | Assay Description Concentration tested in vitro to inhibit PAF-induced maximum aggregation (PAF-Antagonistic activity) by 50%. | J Med Chem 35: 4118-34 (1992) BindingDB Entry DOI: 10.7270/Q228087F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131619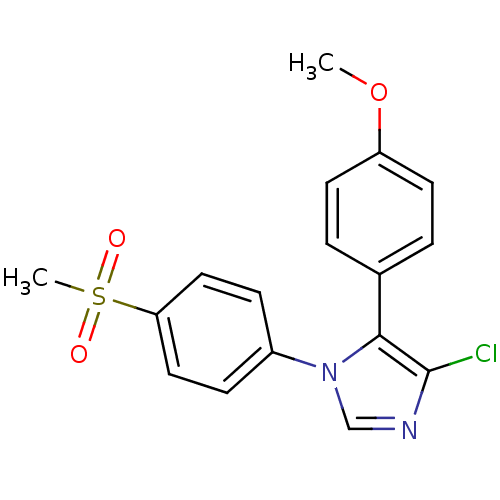 (4-Chloro-1-(4-methanesulfonyl-phenyl)-5-(4-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50045982 (CHEMBL103596 | [4-(3-Methylamino-3-phenyl-propiony...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía, S.A. Curated by ChEMBL | Assay Description Inhibition of PAF-induced platelet aggregation | J Med Chem 36: 2984-97 (1993) BindingDB Entry DOI: 10.7270/Q2ZS2VKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50005232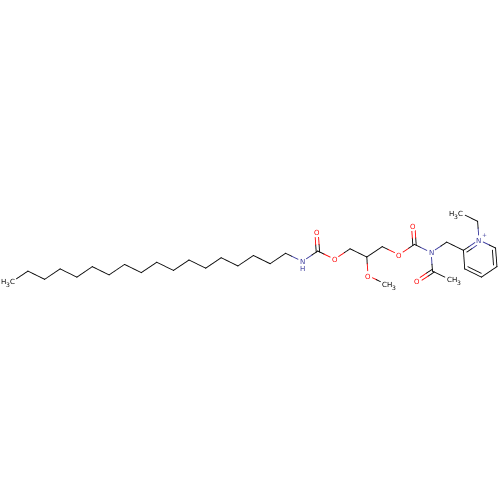 ((R)-2-{[Acetyl-(2-methoxy-3-octadecylcarbamoyloxy-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
J. Uriach & Cía.S.A. Curated by ChEMBL | Assay Description Inhibitory activity expressed as the concentration required to inhibit platelet-activating factor (PAF) induced maximum aggregation by 50% | J Med Chem 34: 373-86 (1991) BindingDB Entry DOI: 10.7270/Q2DF6Q51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50131588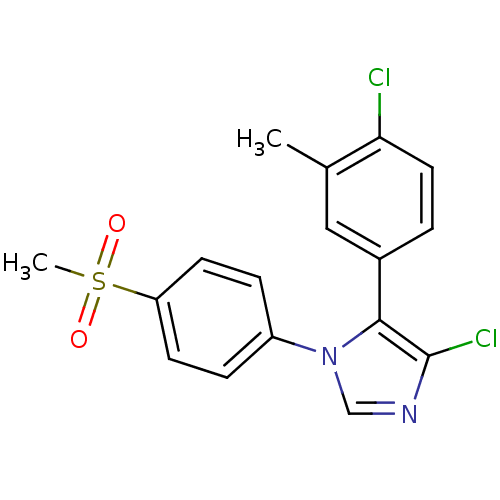 (4-Chloro-5-(4-chloro-3-methyl-phenyl)-1-(4-methane...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Domp&egrove; pha.r.ma s.p.a. Curated by ChEMBL | Assay Description In vitro percent inhibition of Prostaglandin G/H synthase 2 (COX-2) in human whole blood was determined | J Med Chem 46: 3463-75 (2003) Article DOI: 10.1021/jm030765s BindingDB Entry DOI: 10.7270/Q2HQ3Z98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 461 total ) | Next | Last >> |