 Found 173 hits with Last Name = 'kallin' and Initial = 'e'
Found 173 hits with Last Name = 'kallin' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466897
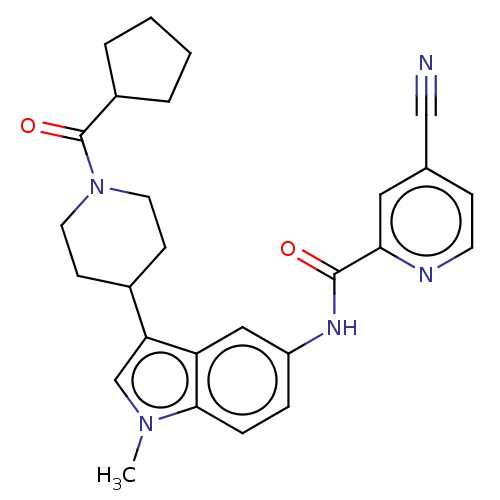
(CHEMBL4287715)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cc(ccn3)C#N)ccc12 Show InChI InChI=1S/C27H29N5O2/c1-31-17-23(19-9-12-32(13-10-19)27(34)20-4-2-3-5-20)22-15-21(6-7-25(22)31)30-26(33)24-14-18(16-28)8-11-29-24/h6-8,11,14-15,17,19-20H,2-5,9-10,12-13H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466891
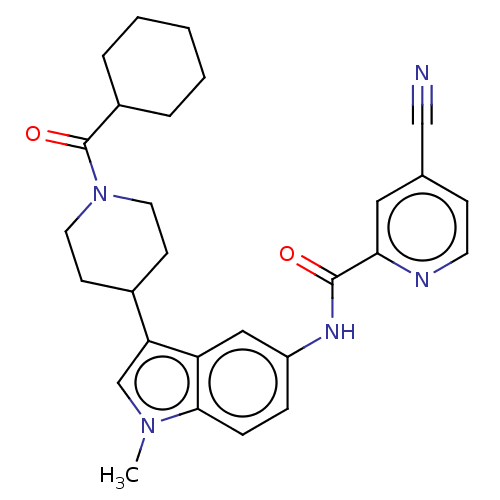
(CHEMBL4281109)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCCC2)c2cc(NC(=O)c3cc(ccn3)C#N)ccc12 Show InChI InChI=1S/C28H31N5O2/c1-32-18-24(20-10-13-33(14-11-20)28(35)21-5-3-2-4-6-21)23-16-22(7-8-26(23)32)31-27(34)25-15-19(17-29)9-12-30-25/h7-9,12,15-16,18,20-21H,2-6,10-11,13-14H2,1H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466893
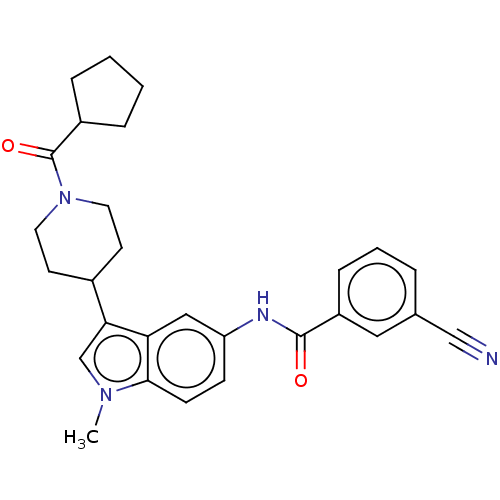
(CHEMBL4291727)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cccc(c3)C#N)ccc12 Show InChI InChI=1S/C28H30N4O2/c1-31-18-25(20-11-13-32(14-12-20)28(34)21-6-2-3-7-21)24-16-23(9-10-26(24)31)30-27(33)22-8-4-5-19(15-22)17-29/h4-5,8-10,15-16,18,20-21H,2-3,6-7,11-14H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466892
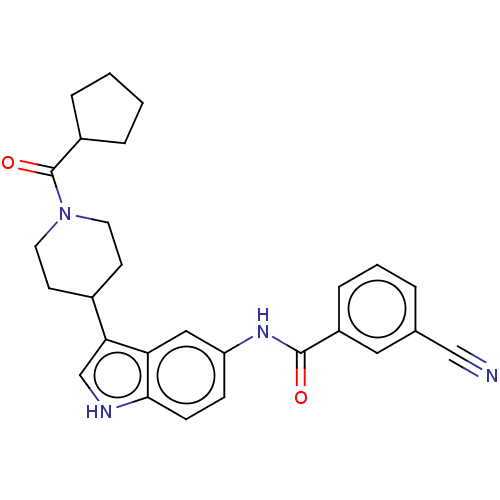
(CHEMBL4283871)Show SMILES O=C(Nc1ccc2[nH]cc(C3CCN(CC3)C(=O)C3CCCC3)c2c1)c1cccc(c1)C#N Show InChI InChI=1S/C27H28N4O2/c28-16-18-4-3-7-21(14-18)26(32)30-22-8-9-25-23(15-22)24(17-29-25)19-10-12-31(13-11-19)27(33)20-5-1-2-6-20/h3-4,7-9,14-15,17,19-20,29H,1-2,5-6,10-13H2,(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466913

(CHEMBL4289304)Show SMILES CCn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cc(ccn3)C#N)ccc12 Show InChI InChI=1S/C28H31N5O2/c1-2-32-18-24(20-10-13-33(14-11-20)28(35)21-5-3-4-6-21)23-16-22(7-8-26(23)32)31-27(34)25-15-19(17-29)9-12-30-25/h7-9,12,15-16,18,20-21H,2-6,10-11,13-14H2,1H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50172920
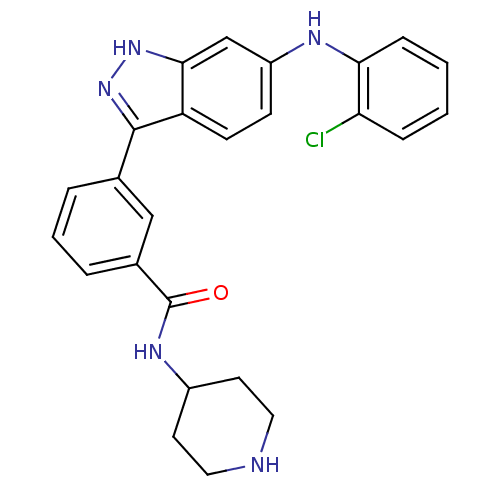
(3-(6-(2-chlorophenylamino)-1H-indazol-3-yl)-N-(pip...)Show SMILES Clc1ccccc1Nc1ccc2c(n[nH]c2c1)-c1cccc(c1)C(=O)NC1CCNCC1 Show InChI InChI=1S/C25H24ClN5O/c26-21-6-1-2-7-22(21)28-19-8-9-20-23(15-19)30-31-24(20)16-4-3-5-17(14-16)25(32)29-18-10-12-27-13-11-18/h1-9,14-15,18,27-28H,10-13H2,(H,29,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibitory concentration against c-Jun N-terminal kinase 3 |
Bioorg Med Chem Lett 15: 5095-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.083
BindingDB Entry DOI: 10.7270/Q2JW8DFC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50211306
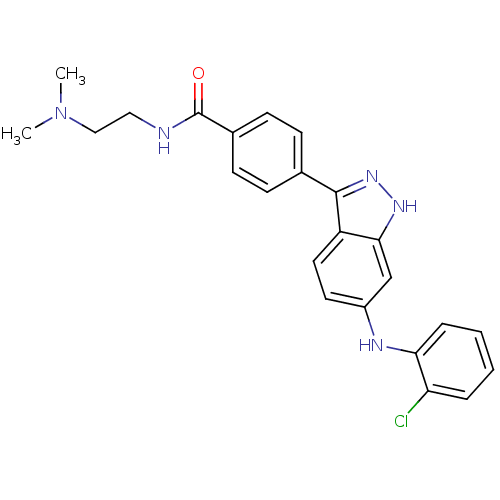
(4-(6-(2-chlorophenylamino)-1H-indazol-3-yl)-N-(2-(...)Show SMILES CN(C)CCNC(=O)c1ccc(cc1)-c1n[nH]c2cc(Nc3ccccc3Cl)ccc12 Show InChI InChI=1S/C24H24ClN5O/c1-30(2)14-13-26-24(31)17-9-7-16(8-10-17)23-19-12-11-18(15-22(19)28-29-23)27-21-6-4-3-5-20(21)25/h3-12,15,27H,13-14H2,1-2H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibitory concentration against c-Jun N-terminal kinase 3 |
Bioorg Med Chem Lett 15: 5095-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.083
BindingDB Entry DOI: 10.7270/Q2JW8DFC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178827

((+)-N-(4-(2-(4-fluorophenylamino)pyridin-4-yl)pyri...)Show SMILES Fc1ccc(Nc2cc(ccn2)-c2ccnc(NC(=O)C3CCOC3)c2)cc1 Show InChI InChI=1S/C21H19FN4O2/c22-17-1-3-18(4-2-17)25-19-11-14(5-8-23-19)15-6-9-24-20(12-15)26-21(27)16-7-10-28-13-16/h1-6,8-9,11-12,16H,7,10,13H2,(H,23,25)(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 1397-401 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.039
BindingDB Entry DOI: 10.7270/Q2KP81R3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178827

((+)-N-(4-(2-(4-fluorophenylamino)pyridin-4-yl)pyri...)Show SMILES Fc1ccc(Nc2cc(ccn2)-c2ccnc(NC(=O)C3CCOC3)c2)cc1 Show InChI InChI=1S/C21H19FN4O2/c22-17-1-3-18(4-2-17)25-19-11-14(5-8-23-19)15-6-9-24-20(12-15)26-21(27)16-7-10-28-13-16/h1-6,8-9,11-12,16H,7,10,13H2,(H,23,25)(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 1397-401 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.039
BindingDB Entry DOI: 10.7270/Q2KP81R3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50172919
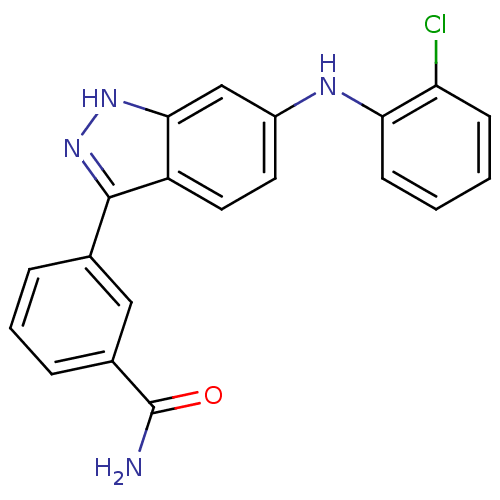
(3-[6-(2-Chloro-phenylamino)-1H-indazol-3-yl]-benza...)Show SMILES NC(=O)c1cccc(c1)-c1n[nH]c2cc(Nc3ccccc3Cl)ccc12 Show InChI InChI=1S/C20H15ClN4O/c21-16-6-1-2-7-17(16)23-14-8-9-15-18(11-14)24-25-19(15)12-4-3-5-13(10-12)20(22)26/h1-11,23H,(H2,22,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 15: 5095-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.083
BindingDB Entry DOI: 10.7270/Q2JW8DFC |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466890
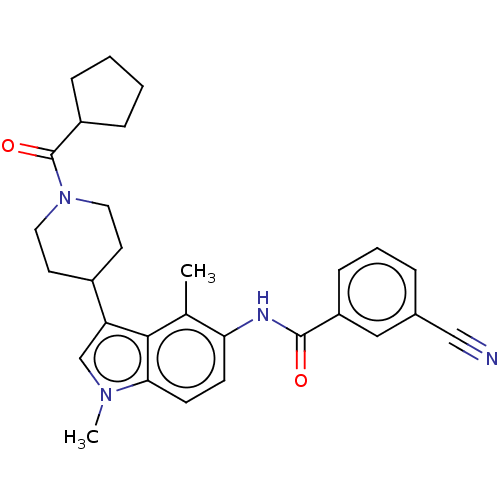
(CHEMBL4290728)Show SMILES Cc1c(NC(=O)c2cccc(c2)C#N)ccc2n(C)cc(C3CCN(CC3)C(=O)C3CCCC3)c12 Show InChI InChI=1S/C29H32N4O2/c1-19-25(31-28(34)23-9-5-6-20(16-23)17-30)10-11-26-27(19)24(18-32(26)2)21-12-14-33(15-13-21)29(35)22-7-3-4-8-22/h5-6,9-11,16,18,21-22H,3-4,7-8,12-15H2,1-2H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISA |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50172919

(3-[6-(2-Chloro-phenylamino)-1H-indazol-3-yl]-benza...)Show SMILES NC(=O)c1cccc(c1)-c1n[nH]c2cc(Nc3ccccc3Cl)ccc12 Show InChI InChI=1S/C20H15ClN4O/c21-16-6-1-2-7-17(16)23-14-8-9-15-18(11-14)24-25-19(15)12-4-3-5-13(10-12)20(22)26/h1-11,23H,(H2,22,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibitory concentration against c-Jun N-terminal kinase 3 |
Bioorg Med Chem Lett 15: 5095-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.083
BindingDB Entry DOI: 10.7270/Q2JW8DFC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50172921
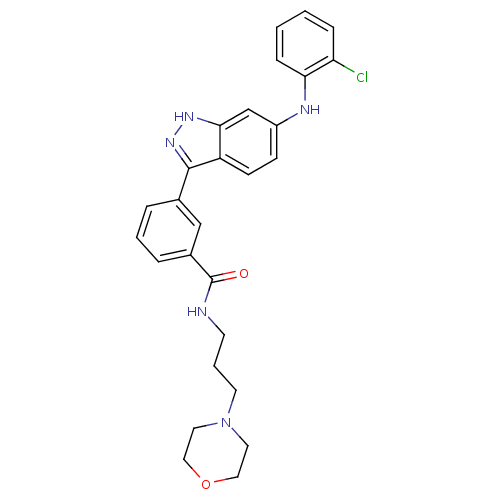
(3-[6-(2-Chloro-phenylamino)-1H-indazol-3-yl]-N-(3-...)Show SMILES Clc1ccccc1Nc1ccc2c(n[nH]c2c1)-c1cccc(c1)C(=O)NCCCN1CCOCC1 Show InChI InChI=1S/C27H28ClN5O2/c28-23-7-1-2-8-24(23)30-21-9-10-22-25(18-21)31-32-26(22)19-5-3-6-20(17-19)27(34)29-11-4-12-33-13-15-35-16-14-33/h1-3,5-10,17-18,30H,4,11-16H2,(H,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibitory concentration against c-Jun N-terminal kinase 3 |
Bioorg Med Chem Lett 15: 5095-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.083
BindingDB Entry DOI: 10.7270/Q2JW8DFC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50172921

(3-[6-(2-Chloro-phenylamino)-1H-indazol-3-yl]-N-(3-...)Show SMILES Clc1ccccc1Nc1ccc2c(n[nH]c2c1)-c1cccc(c1)C(=O)NCCCN1CCOCC1 Show InChI InChI=1S/C27H28ClN5O2/c28-23-7-1-2-8-24(23)30-21-9-10-22-25(18-21)31-32-26(22)19-5-3-6-20(17-19)27(34)29-11-4-12-33-13-15-35-16-14-33/h1-3,5-10,17-18,30H,4,11-16H2,(H,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 15: 5095-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.083
BindingDB Entry DOI: 10.7270/Q2JW8DFC |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM189877

(US10227346, Example 5 | US10426135, Example 5 | US...)Show SMILES CC(C)C(=O)N1CCC(CC1)c1cn(C)c2ncc(NC(=O)c3cccc(c3)C#N)c(c12)C(F)(F)F Show InChI InChI=1S/C26H26F3N5O2/c1-15(2)25(36)34-9-7-17(8-10-34)19-14-33(3)23-21(19)22(26(27,28)29)20(13-31-23)32-24(35)18-6-4-5-16(11-18)12-30/h4-6,11,13-15,17H,7-10H2,1-3H3,(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50172920

(3-(6-(2-chlorophenylamino)-1H-indazol-3-yl)-N-(pip...)Show SMILES Clc1ccccc1Nc1ccc2c(n[nH]c2c1)-c1cccc(c1)C(=O)NC1CCNCC1 Show InChI InChI=1S/C25H24ClN5O/c26-21-6-1-2-7-22(21)28-19-8-9-20-23(15-19)30-31-24(20)16-4-3-5-17(14-16)25(32)29-18-10-12-27-13-11-18/h1-9,14-15,18,27-28H,10-13H2,(H,29,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 15: 5095-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.083
BindingDB Entry DOI: 10.7270/Q2JW8DFC |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466912

(CHEMBL4283051)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cc(ccn3)C#N)cnc12 Show InChI InChI=1S/C26H28N6O2/c1-31-16-22(18-7-10-32(11-8-18)26(34)19-4-2-3-5-19)21-13-20(15-29-24(21)31)30-25(33)23-12-17(14-27)6-9-28-23/h6,9,12-13,15-16,18-19H,2-5,7-8,10-11H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466897

(CHEMBL4287715)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cc(ccn3)C#N)ccc12 Show InChI InChI=1S/C27H29N5O2/c1-31-17-23(19-9-12-32(13-10-19)27(34)20-4-2-3-5-20)22-15-21(6-7-25(22)31)30-26(33)24-14-18(16-28)8-11-29-24/h6-8,11,14-15,17,19-20H,2-5,9-10,12-13H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178843
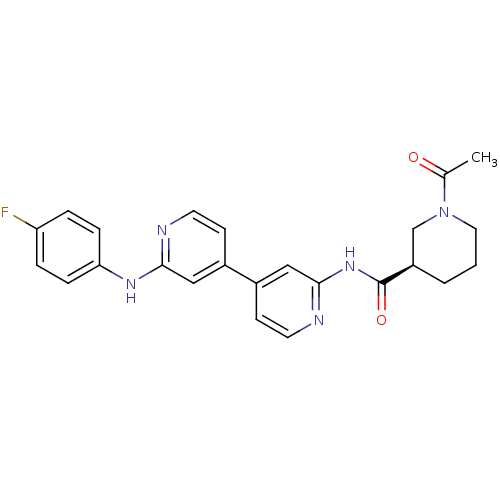
((3R)-1-acetyl-N-(4-(2-(4-fluorophenylamino)pyridin...)Show SMILES CC(=O)N1CCC[C@H](C1)C(=O)Nc1cc(ccn1)-c1ccnc(Nc2ccc(F)cc2)c1 Show InChI InChI=1S/C24H24FN5O2/c1-16(31)30-12-2-3-19(15-30)24(32)29-23-14-18(9-11-27-23)17-8-10-26-22(13-17)28-21-6-4-20(25)5-7-21/h4-11,13-14,19H,2-3,12,15H2,1H3,(H,26,28)(H,27,29,32)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 1397-401 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.039
BindingDB Entry DOI: 10.7270/Q2KP81R3 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466891

(CHEMBL4281109)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCCC2)c2cc(NC(=O)c3cc(ccn3)C#N)ccc12 Show InChI InChI=1S/C28H31N5O2/c1-32-18-24(20-10-13-33(14-11-20)28(35)21-5-3-2-4-6-21)23-16-22(7-8-26(23)32)31-27(34)25-15-19(17-29)9-12-30-25/h7-9,12,15-16,18,20-21H,2-6,10-11,13-14H2,1H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466890

(CHEMBL4290728)Show SMILES Cc1c(NC(=O)c2cccc(c2)C#N)ccc2n(C)cc(C3CCN(CC3)C(=O)C3CCCC3)c12 Show InChI InChI=1S/C29H32N4O2/c1-19-25(31-28(34)23-9-5-6-20(16-23)17-30)10-11-26-27(19)24(18-32(26)2)21-12-14-33(15-13-21)29(35)22-7-3-4-8-22/h5-6,9-11,16,18,21-22H,3-4,7-8,12-15H2,1-2H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50172927
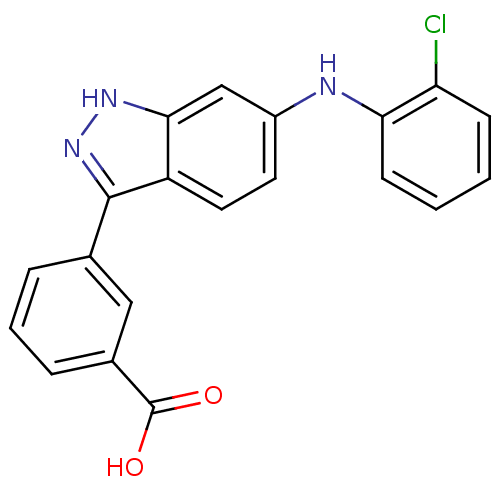
(3-[6-(2-Chloro-phenylamino)-1H-indazol-3-yl]-benzo...)Show SMILES OC(=O)c1cccc(c1)-c1n[nH]c2cc(Nc3ccccc3Cl)ccc12 Show InChI InChI=1S/C20H14ClN3O2/c21-16-6-1-2-7-17(16)22-14-8-9-15-18(11-14)23-24-19(15)12-4-3-5-13(10-12)20(25)26/h1-11,22H,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibitory concentration against c-Jun N-terminal kinase 3 |
Bioorg Med Chem Lett 15: 5095-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.083
BindingDB Entry DOI: 10.7270/Q2JW8DFC |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466893

(CHEMBL4291727)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cccc(c3)C#N)ccc12 Show InChI InChI=1S/C28H30N4O2/c1-31-18-25(20-11-13-32(14-12-20)28(34)21-6-2-3-7-21)24-16-23(9-10-26(24)31)30-27(33)22-8-4-5-19(15-22)17-29/h4-5,8-10,15-16,18,20-21H,2-3,6-7,11-14H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178830

(CHEMBL205078 | N-(4-(2-(4-fluorophenylamino)pyridi...)Show SMILES Fc1ccc(Nc2cc(ccn2)-c2ccnc(NC(=O)C3CCOCC3)c2)cc1 Show InChI InChI=1S/C22H21FN4O2/c23-18-1-3-19(4-2-18)26-20-13-16(5-9-24-20)17-6-10-25-21(14-17)27-22(28)15-7-11-29-12-8-15/h1-6,9-10,13-15H,7-8,11-12H2,(H,24,26)(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 1397-401 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.039
BindingDB Entry DOI: 10.7270/Q2KP81R3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178832
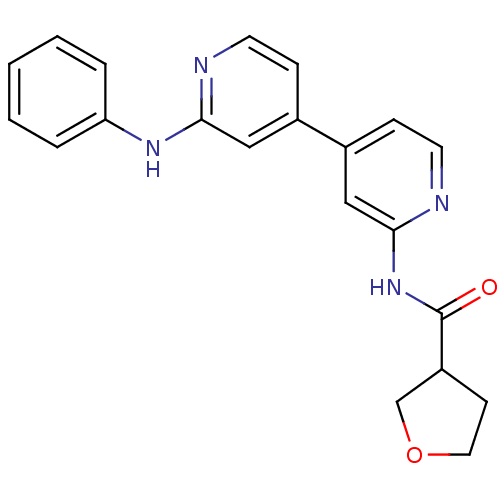
(CHEMBL203535 | N-(2'-(phenylamino)-4,4'-bipyridin-...)Show SMILES O=C(Nc1cc(ccn1)-c1ccnc(Nc2ccccc2)c1)C1CCOC1 Show InChI InChI=1S/C21H20N4O2/c26-21(17-8-11-27-14-17)25-20-13-16(7-10-23-20)15-6-9-22-19(12-15)24-18-4-2-1-3-5-18/h1-7,9-10,12-13,17H,8,11,14H2,(H,22,24)(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 1397-401 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.039
BindingDB Entry DOI: 10.7270/Q2KP81R3 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466914

(CHEMBL4283312)Show SMILES CC(C)C(=O)N1CCC(CC1)c1cn(C)c2ncc(NC(=O)c3ccc(Cl)c(c3)C#N)c(C)c12 Show InChI InChI=1S/C26H28ClN5O2/c1-15(2)26(34)32-9-7-17(8-10-32)20-14-31(4)24-23(20)16(3)22(13-29-24)30-25(33)18-5-6-21(27)19(11-18)12-28/h5-6,11,13-15,17H,7-10H2,1-4H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466915

(CHEMBL4282848)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2cc(NC(=O)c3cccc(c3)C#N)cnc12 Show InChI InChI=1S/C27H29N5O2/c1-31-17-24(19-9-11-32(12-10-19)27(34)20-6-2-3-7-20)23-14-22(16-29-25(23)31)30-26(33)21-8-4-5-18(13-21)15-28/h4-5,8,13-14,16-17,19-20H,2-3,6-7,9-12H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178836
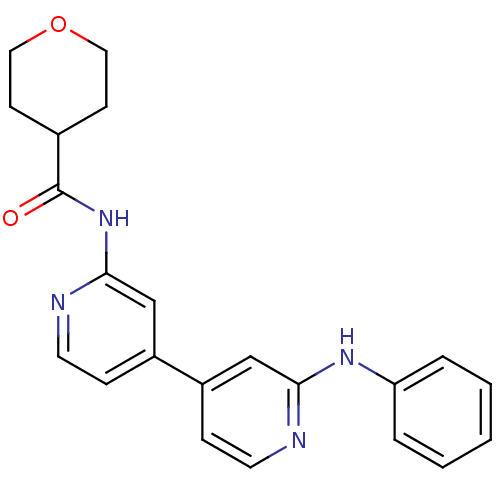
(CHEMBL203059 | N-(4-(2-(phenylamino)pyridin-4-yl)p...)Show SMILES O=C(Nc1cc(ccn1)-c1ccnc(Nc2ccccc2)c1)C1CCOCC1 Show InChI InChI=1S/C22H22N4O2/c27-22(16-8-12-28-13-9-16)26-21-15-18(7-11-24-21)17-6-10-23-20(14-17)25-19-4-2-1-3-5-19/h1-7,10-11,14-16H,8-9,12-13H2,(H,23,25)(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 1397-401 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.039
BindingDB Entry DOI: 10.7270/Q2KP81R3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50211306

(4-(6-(2-chlorophenylamino)-1H-indazol-3-yl)-N-(2-(...)Show SMILES CN(C)CCNC(=O)c1ccc(cc1)-c1n[nH]c2cc(Nc3ccccc3Cl)ccc12 Show InChI InChI=1S/C24H24ClN5O/c1-30(2)14-13-26-24(31)17-9-7-16(8-10-17)23-19-12-11-18(15-22(19)28-29-23)27-21-6-4-3-5-20(21)25/h3-12,15,27H,13-14H2,1-2H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 15: 5095-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.083
BindingDB Entry DOI: 10.7270/Q2JW8DFC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178846

(1-acetyl-N-(4-(2-(phenylamino)pyridin-4-yl)pyridin...)Show SMILES CC(=O)N1CCCC(C1)C(=O)Nc1cc(ccn1)-c1ccnc(Nc2ccccc2)c1 Show InChI InChI=1S/C24H25N5O2/c1-17(30)29-13-5-6-20(16-29)24(31)28-23-15-19(10-12-26-23)18-9-11-25-22(14-18)27-21-7-3-2-4-8-21/h2-4,7-12,14-15,20H,5-6,13,16H2,1H3,(H,25,27)(H,26,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 1397-401 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.039
BindingDB Entry DOI: 10.7270/Q2KP81R3 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM189877

(US10227346, Example 5 | US10426135, Example 5 | US...)Show SMILES CC(C)C(=O)N1CCC(CC1)c1cn(C)c2ncc(NC(=O)c3cccc(c3)C#N)c(c12)C(F)(F)F Show InChI InChI=1S/C26H26F3N5O2/c1-15(2)25(36)34-9-7-17(8-10-34)19-14-33(3)23-21(19)22(26(27,28)29)20(13-31-23)32-24(35)18-6-4-5-16(11-18)12-30/h4-6,11,13-15,17H,7-10H2,1-3H3,(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISA |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466908

(CHEMBL4283530)Show SMILES Cc1c(NC(=O)c2cccc(c2)C#N)cnc2n(C)cc(C3CCN(CC3)C(=O)C3CCCCC3)c12 Show InChI InChI=1S/C29H33N5O2/c1-19-25(32-28(35)23-10-6-7-20(15-23)16-30)17-31-27-26(19)24(18-33(27)2)21-11-13-34(14-12-21)29(36)22-8-4-3-5-9-22/h6-7,10,15,17-18,21-22H,3-5,8-9,11-14H2,1-2H3,(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM189922

(US10227346, Example 109 | US10426135, Example 109 ...)Show SMILES CC(C)CC(=O)N1CCC(CC1)c1cn(C)c2ncc(NC(=O)c3cccc(c3)C#N)c(C)c12 Show InChI InChI=1S/C27H31N5O2/c1-17(2)12-24(33)32-10-8-20(9-11-32)22-16-31(4)26-25(22)18(3)23(15-29-26)30-27(34)21-7-5-6-19(13-21)14-28/h5-7,13,15-17,20H,8-12H2,1-4H3,(H,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178842
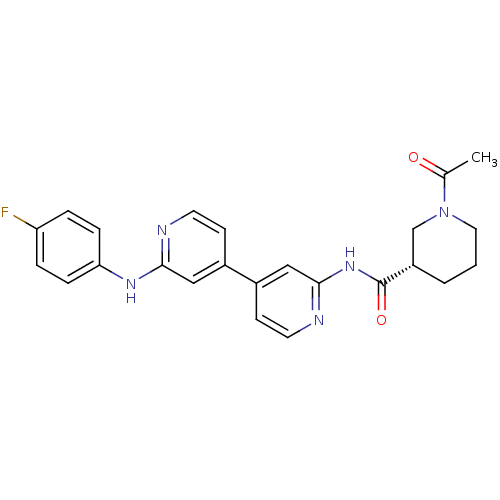
((3S)-1-acetyl-N-(4-(2-(4-fluorophenylamino)pyridin...)Show SMILES CC(=O)N1CCC[C@@H](C1)C(=O)Nc1cc(ccn1)-c1ccnc(Nc2ccc(F)cc2)c1 Show InChI InChI=1S/C24H24FN5O2/c1-16(31)30-12-2-3-19(15-30)24(32)29-23-14-18(9-11-27-23)17-8-10-26-22(13-17)28-21-6-4-20(25)5-7-21/h4-11,13-14,19H,2-3,12,15H2,1H3,(H,26,28)(H,27,29,32)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 1397-401 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.039
BindingDB Entry DOI: 10.7270/Q2KP81R3 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466895

(CHEMBL4281170)Show SMILES Cc1c(NC(=O)c2cccc(c2)C#N)cnc2n(C)cc(C3CCN(CC3)C(=O)C3CCC3)c12 Show InChI InChI=1S/C27H29N5O2/c1-17-23(30-26(33)21-8-3-5-18(13-21)14-28)15-29-25-24(17)22(16-31(25)2)19-9-11-32(12-10-19)27(34)20-6-4-7-20/h3,5,8,13,15-16,19-20H,4,6-7,9-12H2,1-2H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50172922
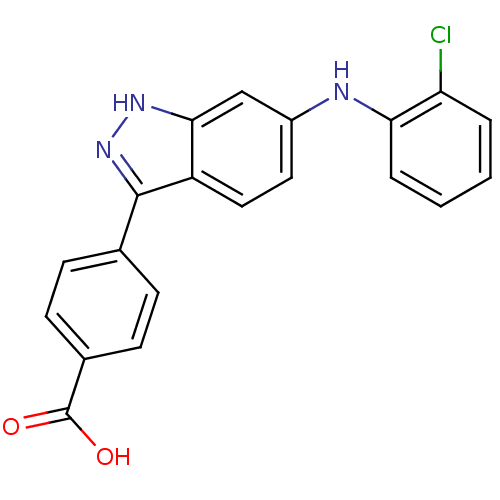
(4-[6-(2-Chloro-phenylamino)-1H-indazol-3-yl]-benzo...)Show SMILES OC(=O)c1ccc(cc1)-c1n[nH]c2cc(Nc3ccccc3Cl)ccc12 Show InChI InChI=1S/C20H14ClN3O2/c21-16-3-1-2-4-17(16)22-14-9-10-15-18(11-14)23-24-19(15)12-5-7-13(8-6-12)20(25)26/h1-11,22H,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 15: 5095-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.083
BindingDB Entry DOI: 10.7270/Q2JW8DFC |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466909
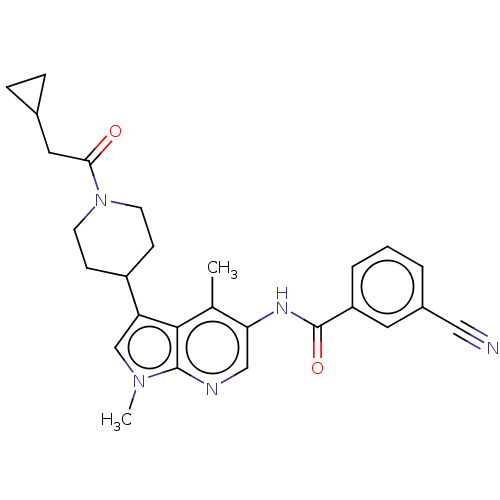
(CHEMBL4284588)Show SMILES Cc1c(NC(=O)c2cccc(c2)C#N)cnc2n(C)cc(C3CCN(CC3)C(=O)CC3CC3)c12 Show InChI InChI=1S/C27H29N5O2/c1-17-23(30-27(34)21-5-3-4-19(12-21)14-28)15-29-26-25(17)22(16-31(26)2)20-8-10-32(11-9-20)24(33)13-18-6-7-18/h3-5,12,15-16,18,20H,6-11,13H2,1-2H3,(H,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466911
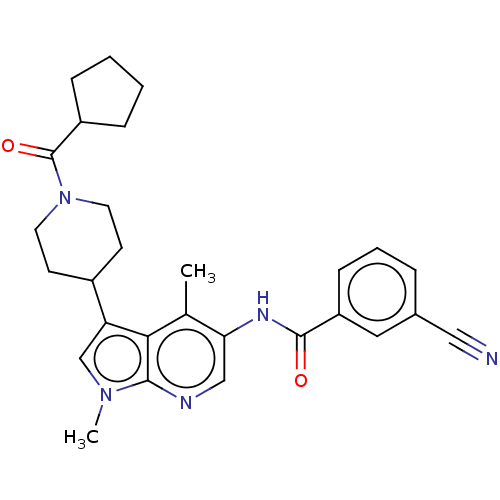
(CHEMBL4288376)Show SMILES Cc1c(NC(=O)c2cccc(c2)C#N)cnc2n(C)cc(C3CCN(CC3)C(=O)C3CCCC3)c12 Show InChI InChI=1S/C28H31N5O2/c1-18-24(31-27(34)22-9-5-6-19(14-22)15-29)16-30-26-25(18)23(17-32(26)2)20-10-12-33(13-11-20)28(35)21-7-3-4-8-21/h5-6,9,14,16-17,20-21H,3-4,7-8,10-13H2,1-2H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466908

(CHEMBL4283530)Show SMILES Cc1c(NC(=O)c2cccc(c2)C#N)cnc2n(C)cc(C3CCN(CC3)C(=O)C3CCCCC3)c12 Show InChI InChI=1S/C29H33N5O2/c1-19-25(32-28(35)23-10-6-7-20(15-23)16-30)17-31-27-26(19)24(18-33(27)2)21-11-13-34(14-12-21)29(36)22-8-4-3-5-9-22/h6-7,10,15,17-18,21-22H,3-5,8-9,11-14H2,1-2H3,(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISA |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM189909

(US10227346, Example 33 | US10426135, Example 33 | ...)Show SMILES Cc1cccc(F)c1C(=O)N1CCC(CC1)c1cn(C)c2ncc(NC(=O)c3cccc(c3)C#N)c(C)c12 Show InChI InChI=1S/C30H28FN5O2/c1-18-6-4-9-24(31)26(18)30(38)36-12-10-21(11-13-36)23-17-35(3)28-27(23)19(2)25(16-33-28)34-29(37)22-8-5-7-20(14-22)15-32/h4-9,14,16-17,21H,10-13H2,1-3H3,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISA |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466907

(CHEMBL4291498)Show SMILES CC(C)C(=O)N1CCC(CC1)c1cn(C)c2ncc(NC(=O)c3ccc(C)c(c3)C#N)c(C)c12 Show InChI InChI=1S/C27H31N5O2/c1-16(2)27(34)32-10-8-19(9-11-32)22-15-31(5)25-24(22)18(4)23(14-29-25)30-26(33)20-7-6-17(3)21(12-20)13-28/h6-7,12,14-16,19H,8-11H2,1-5H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178839
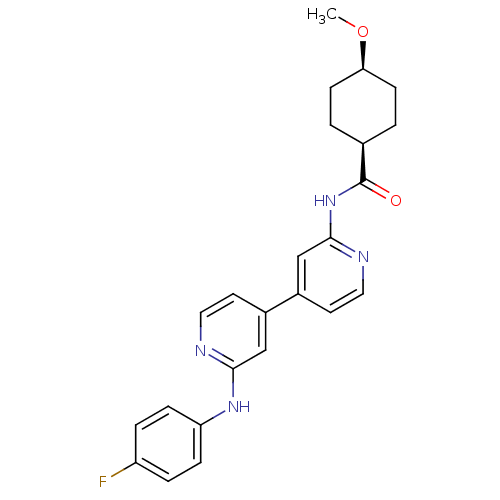
(CHEMBL202676 | cis-N-(4-(2-(4-fluorophenylamino)py...)Show SMILES CO[C@H]1CC[C@H](CC1)C(=O)Nc1cc(ccn1)-c1ccnc(Nc2ccc(F)cc2)c1 |wU:5.8,2.1,(17.63,-11.64,;17.64,-10.1,;16.3,-9.32,;14.97,-10.09,;13.64,-9.32,;13.64,-7.78,;14.97,-7,;16.31,-7.78,;12.31,-7.01,;12.31,-5.47,;10.98,-7.78,;9.64,-7.01,;9.64,-5.47,;8.31,-4.7,;6.98,-5.46,;6.97,-7.01,;8.31,-7.78,;8.32,-3.16,;9.65,-2.4,;9.66,-.87,;8.33,-.08,;6.99,-.85,;5.66,-.08,;4.32,-.84,;4.33,-2.38,;2.99,-3.15,;1.66,-2.38,;.32,-3.14,;1.67,-.83,;3,-.07,;6.99,-2.39,)| Show InChI InChI=1S/C24H25FN4O2/c1-31-21-8-2-16(3-9-21)24(30)29-23-15-18(11-13-27-23)17-10-12-26-22(14-17)28-20-6-4-19(25)5-7-20/h4-7,10-16,21H,2-3,8-9H2,1H3,(H,26,28)(H,27,29,30)/t16-,21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 1397-401 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.039
BindingDB Entry DOI: 10.7270/Q2KP81R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178838
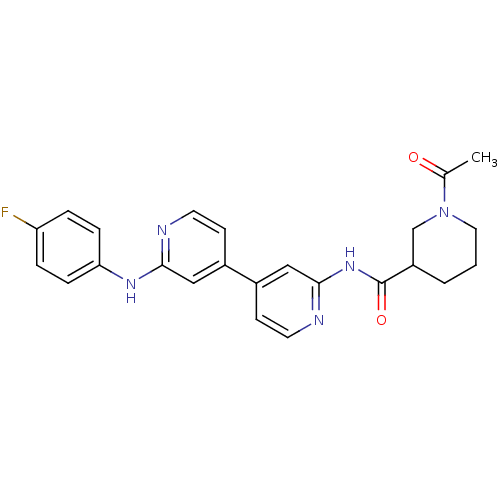
(1-acetyl-N-(4-(2-(4-fluorophenylamino)pyridin-4-yl...)Show SMILES CC(=O)N1CCCC(C1)C(=O)Nc1cc(ccn1)-c1ccnc(Nc2ccc(F)cc2)c1 Show InChI InChI=1S/C24H24FN5O2/c1-16(31)30-12-2-3-19(15-30)24(32)29-23-14-18(9-11-27-23)17-8-10-26-22(13-17)28-21-6-4-20(25)5-7-21/h4-11,13-14,19H,2-3,12,15H2,1H3,(H,26,28)(H,27,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 1397-401 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.039
BindingDB Entry DOI: 10.7270/Q2KP81R3 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466910
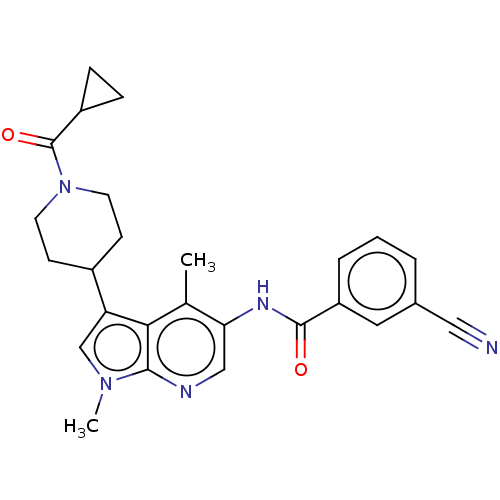
(CHEMBL4292862)Show SMILES Cc1c(NC(=O)c2cccc(c2)C#N)cnc2n(C)cc(C3CCN(CC3)C(=O)C3CC3)c12 Show InChI InChI=1S/C26H27N5O2/c1-16-22(29-25(32)20-5-3-4-17(12-20)13-27)14-28-24-23(16)21(15-30(24)2)18-8-10-31(11-9-18)26(33)19-6-7-19/h3-5,12,14-15,18-19H,6-11H2,1-2H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50172923

(3-[6-(2-Chloro-phenylamino)-1H-indazol-3-yl]-N-(2-...)Show SMILES CN(C)CCNC(=O)c1cccc(c1)-c1n[nH]c2cc(Nc3ccccc3Cl)ccc12 Show InChI InChI=1S/C24H24ClN5O/c1-30(2)13-12-26-24(31)17-7-5-6-16(14-17)23-19-11-10-18(15-22(19)28-29-23)27-21-9-4-3-8-20(21)25/h3-11,14-15,27H,12-13H2,1-2H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 15: 5095-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.083
BindingDB Entry DOI: 10.7270/Q2JW8DFC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178848
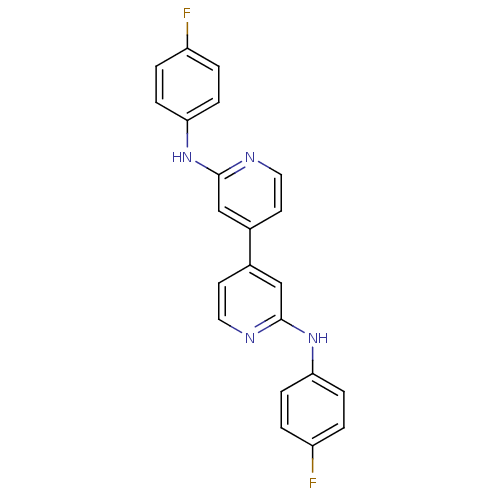
(CHEMBL205672 | N-(4-fluorophenyl)-4-(2-(4-fluoroph...)Show SMILES Fc1ccc(Nc2cc(ccn2)-c2ccnc(Nc3ccc(F)cc3)c2)cc1 Show InChI InChI=1S/C22H16F2N4/c23-17-1-5-19(6-2-17)27-21-13-15(9-11-25-21)16-10-12-26-22(14-16)28-20-7-3-18(24)4-8-20/h1-14H,(H,25,27)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 1397-401 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.039
BindingDB Entry DOI: 10.7270/Q2KP81R3 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM189909

(US10227346, Example 33 | US10426135, Example 33 | ...)Show SMILES Cc1cccc(F)c1C(=O)N1CCC(CC1)c1cn(C)c2ncc(NC(=O)c3cccc(c3)C#N)c(C)c12 Show InChI InChI=1S/C30H28FN5O2/c1-18-6-4-9-24(31)26(18)30(38)36-12-10-21(11-13-36)23-17-35(3)28-27(23)19(2)25(16-33-28)34-29(37)22-8-5-7-20(14-22)15-32/h4-9,14,16-17,21H,10-13H2,1-3H3,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50172918

((2-Chloro-phenyl)-(3-phenyl-1H-indazol-6-yl)-amine...)Show InChI InChI=1S/C19H14ClN3/c20-16-8-4-5-9-17(16)21-14-10-11-15-18(12-14)22-23-19(15)13-6-2-1-3-7-13/h1-12,21H,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 15: 5095-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.083
BindingDB Entry DOI: 10.7270/Q2JW8DFC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178837
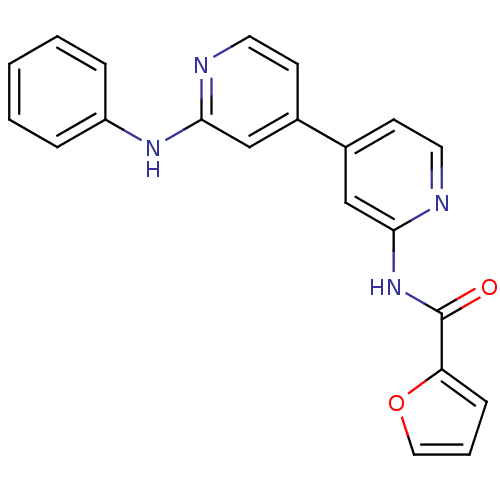
(CHEMBL380724 | N-(4-(2-(phenylamino)pyridin-4-yl)p...)Show SMILES O=C(Nc1cc(ccn1)-c1ccnc(Nc2ccccc2)c1)c1ccco1 Show InChI InChI=1S/C21H16N4O2/c26-21(18-7-4-12-27-18)25-20-14-16(9-11-23-20)15-8-10-22-19(13-15)24-17-5-2-1-3-6-17/h1-14H,(H,22,24)(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 1397-401 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.039
BindingDB Entry DOI: 10.7270/Q2KP81R3 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50466904

(CHEMBL4286366)Show SMILES Cn1cc(C2CCN(CC2)C(=O)C2CCCC2)c2nc(NC(=O)c3cc(ccn3)C#N)ccc12 Show InChI InChI=1S/C26H28N6O2/c1-31-16-20(18-9-12-32(13-10-18)26(34)19-4-2-3-5-19)24-22(31)6-7-23(29-24)30-25(33)21-14-17(15-27)8-11-28-21/h6-8,11,14,16,18-19H,2-5,9-10,12-13H2,1H3,(H,29,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB)
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... |
J Med Chem 61: 10415-10439 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00392
BindingDB Entry DOI: 10.7270/Q27P922T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data