

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017543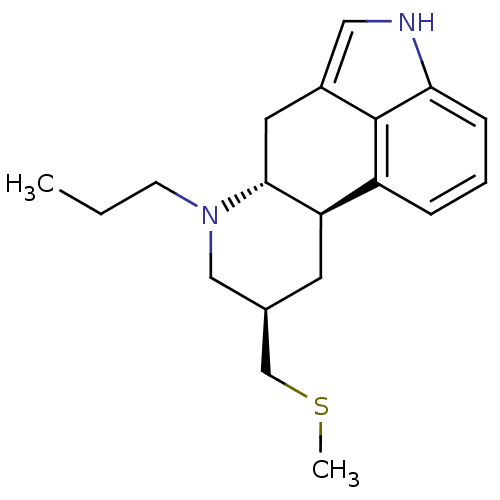 ((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017543 ((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM17051 (BX-795 | BX-795, 3 | N-(3-{[5-iodo-4-({3-[(thiophe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of cGAS in human THP1 cells assessed as reduction in salmon sperm dsDNA-induced IFN-beta expression preincubated for 1 hr followed by dsDN... | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017539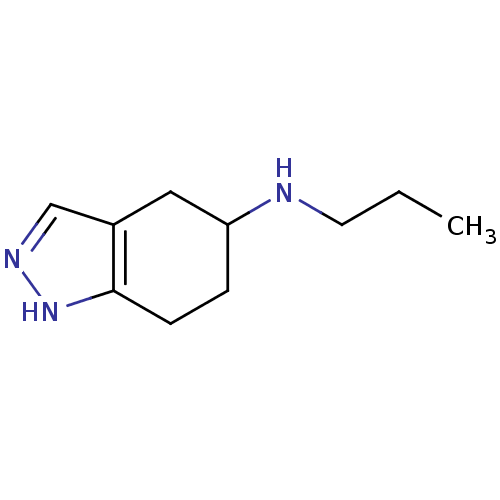 (CHEMBL80414 | Propyl-(4,5,6,7-tetrahydro-2H-indazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017542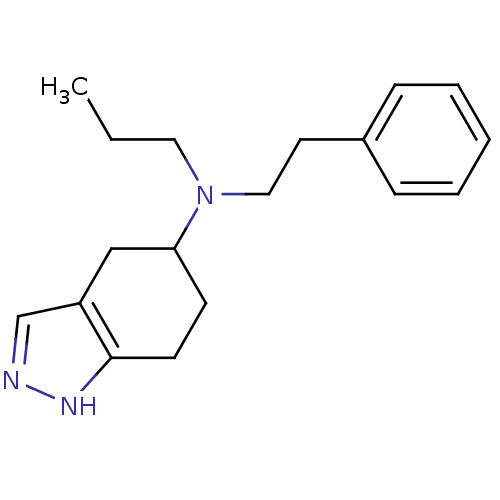 (CHEMBL80437 | Phenethyl-propyl-(4,5,6,7-tetrahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017547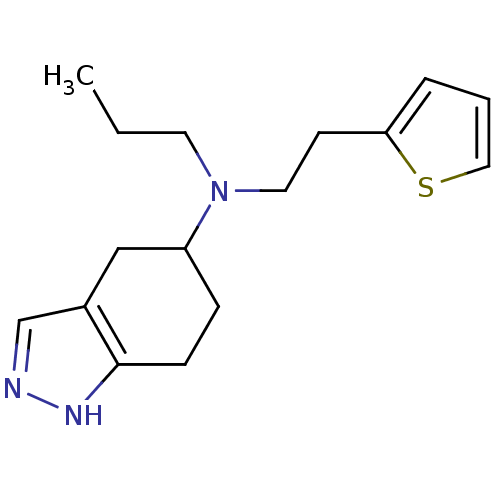 (CHEMBL421512 | Propyl-(4,5,6,7-tetrahydro-2H-indaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017550 (CHEMBL76399 | N*6*-Propyl-4,5,6,7-tetrahydro-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017551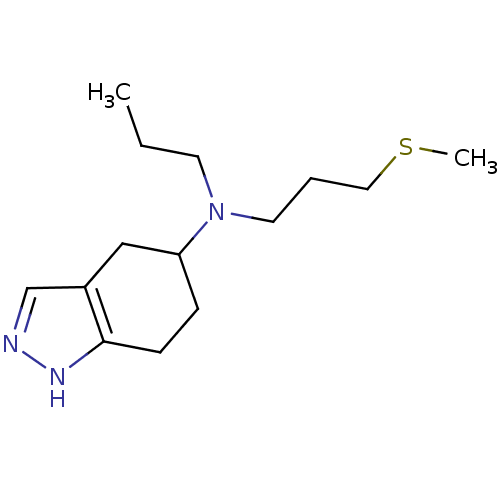 ((3-Methylsulfanyl-propyl)-propyl-(4,5,6,7-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017541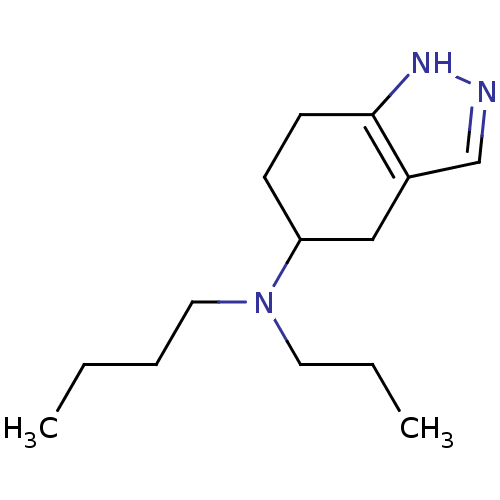 (Butyl-propyl-(4,5,6,7-tetrahydro-2H-indazol-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017544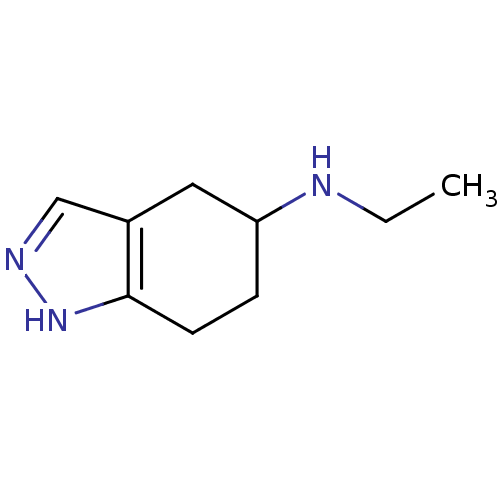 (CHEMBL77421 | Ethyl-(4,5,6,7-tetrahydro-2H-indazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017550 (CHEMBL76399 | N*6*-Propyl-4,5,6,7-tetrahydro-benzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017542 (CHEMBL80437 | Phenethyl-propyl-(4,5,6,7-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017546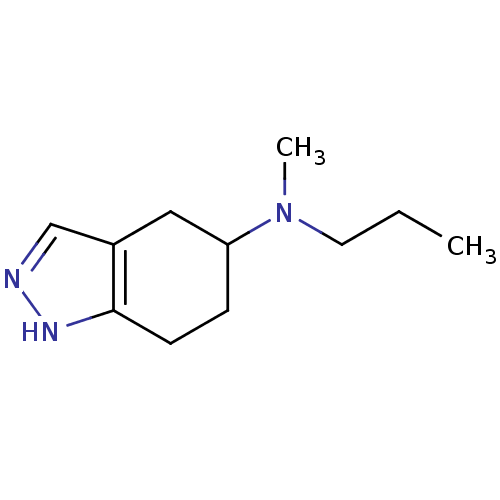 (CHEMBL311225 | Methyl-propyl-(4,5,6,7-tetrahydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017547 (CHEMBL421512 | Propyl-(4,5,6,7-tetrahydro-2H-indaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250108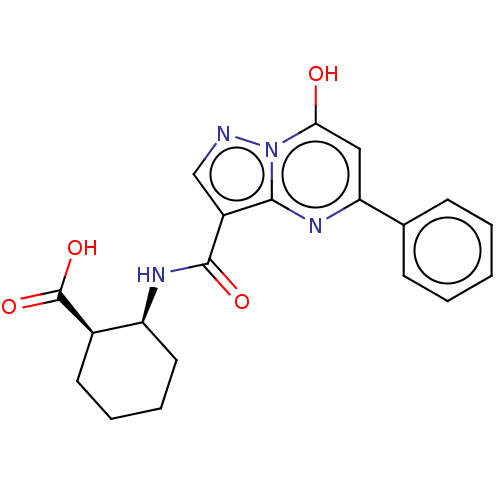 (CHEMBL4096573) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017540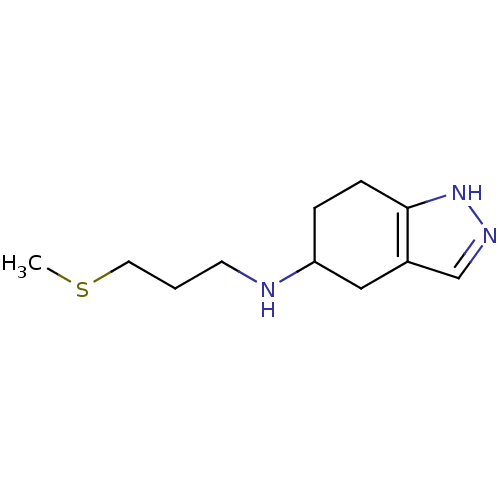 ((3-Methylsulfanyl-propyl)-(4,5,6,7-tetrahydro-2H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017551 ((3-Methylsulfanyl-propyl)-propyl-(4,5,6,7-tetrahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250108 (CHEMBL4096573) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250111 (CHEMBL4084664) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017546 (CHEMBL311225 | Methyl-propyl-(4,5,6,7-tetrahydro-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017545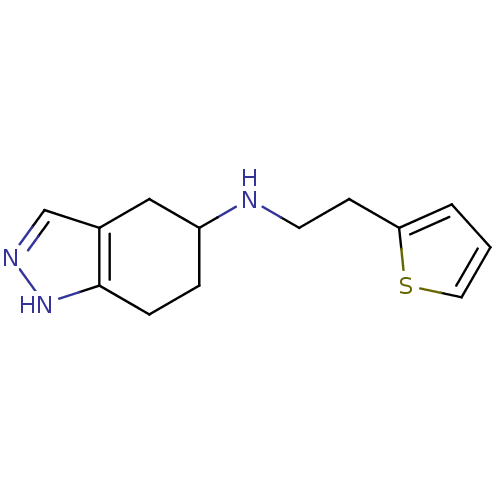 ((4,5,6,7-Tetrahydro-2H-indazol-5-yl)-(2-thiophen-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017549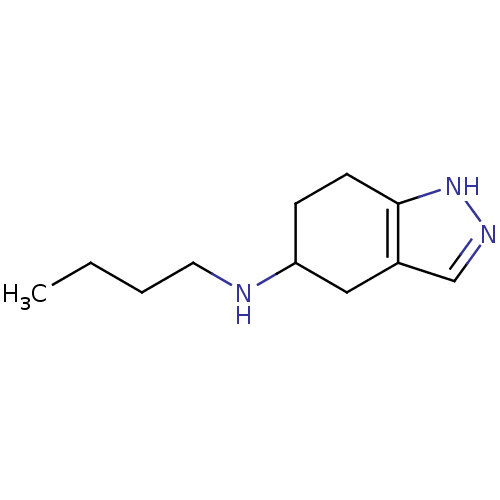 (Butyl-(4,5,6,7-tetrahydro-2H-indazol-5-yl)-amine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017548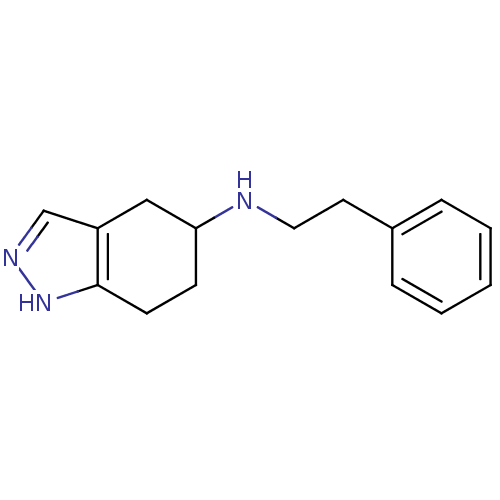 (CHEMBL81400 | Phenethyl-(4,5,6,7-tetrahydro-2H-ind...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017549 (Butyl-(4,5,6,7-tetrahydro-2H-indazol-5-yl)-amine |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017545 ((4,5,6,7-Tetrahydro-2H-indazol-5-yl)-(2-thiophen-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017541 (Butyl-propyl-(4,5,6,7-tetrahydro-2H-indazol-5-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017540 ((3-Methylsulfanyl-propyl)-(4,5,6,7-tetrahydro-2H-i...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250109 (CHEMBL4062994) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017544 (CHEMBL77421 | Ethyl-(4,5,6,7-tetrahydro-2H-indazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250106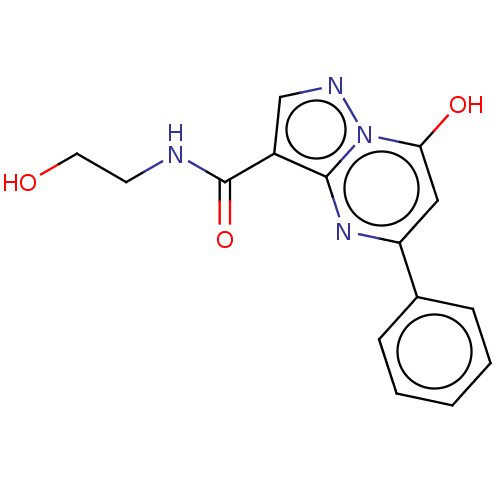 (CHEMBL4103560) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50017539 (CHEMBL80414 | Propyl-(4,5,6,7-tetrahydro-2H-indazo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in calf corpus striatum using [3H]-Spiperone as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017548 (CHEMBL81400 | Phenethyl-(4,5,6,7-tetrahydro-2H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against dopamine receptor D2 in rat corpus striatum using [3H]-Apomorphine as radioligand | J Med Chem 32: 2388-96 (1989) BindingDB Entry DOI: 10.7270/Q2445KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250111 (CHEMBL4084664) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250106 (CHEMBL4103560) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250109 (CHEMBL4062994) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250107 (CHEMBL4085628) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250110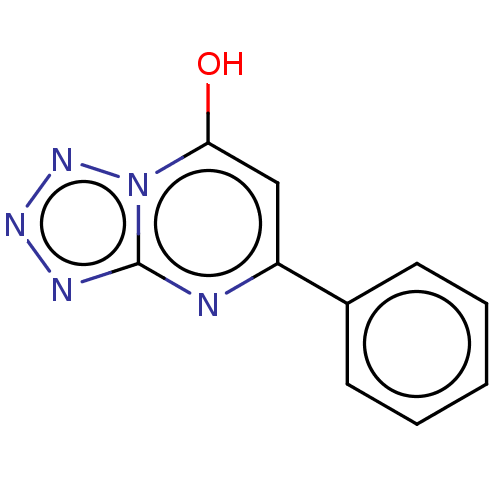 (CHEMBL4065757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250107 (CHEMBL4085628) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human cGAS (2 to 522 residues) expressed in Sf9 insect cells assessed as reduction in cGAMP level using ISD DNA as substrate in presenc... | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250106 (CHEMBL4103560) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human cGAS (2 to 522 residues) expressed in Sf9 insect cells by surface plasmon resonance assay | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250109 (CHEMBL4062994) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human cGAS (2 to 522 residues) expressed in Sf9 insect cells by surface plasmon resonance assay | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250107 (CHEMBL4085628) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human cGAS (2 to 522 residues) expressed in Sf9 insect cells by surface plasmon resonance assay | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250111 (CHEMBL4084664) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human cGAS (2 to 522 residues) expressed in Sf9 insect cells by surface plasmon resonance assay | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250108 (CHEMBL4096573) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human cGAS (2 to 522 residues) expressed in Sf9 insect cells by surface plasmon resonance assay | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclic GMP-AMP synthase (Homo sapiens) | BDBM50250110 (CHEMBL4065757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 1.71E+5 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human cGAS (2 to 522 residues) expressed in Sf9 insect cells by surface plasmon resonance assay | PLoS ONE 12: (2017) Article DOI: 10.1371/journal.pone.0184843 BindingDB Entry DOI: 10.7270/Q2WQ067J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||