

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456708 (CHEMBL4204315) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456708 (CHEMBL4204315) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20079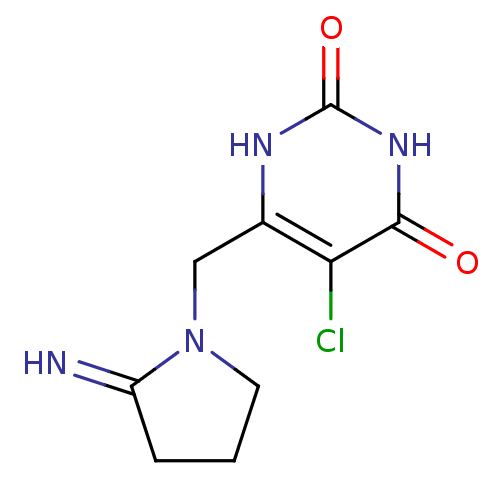 (5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.30 | -52.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456711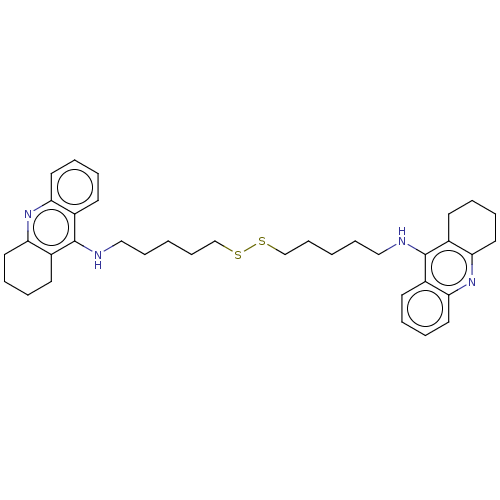 (CHEMBL4208641) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot anal... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456702 (CHEMBL4213042) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456702 (CHEMBL4213042) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456711 (CHEMBL4208641) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456713 (CHEMBL4214235) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot ana... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456712 (CHEMBL4204015) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456712 (CHEMBL4204015) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456710 (CHEMBL4213722) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot ana... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456706 (CHEMBL4217176) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Uncompetitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-B... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456701 (CHEMBL4218651) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456701 (CHEMBL4218651) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456710 (CHEMBL4213722) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweav... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456710 (CHEMBL4213722) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk pl... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456701 (CHEMBL4218651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Bu... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456714 (CHEMBL4217969) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456714 (CHEMBL4217969) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot a... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456703 (CHEMBL4209181) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot ana... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot ana... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456715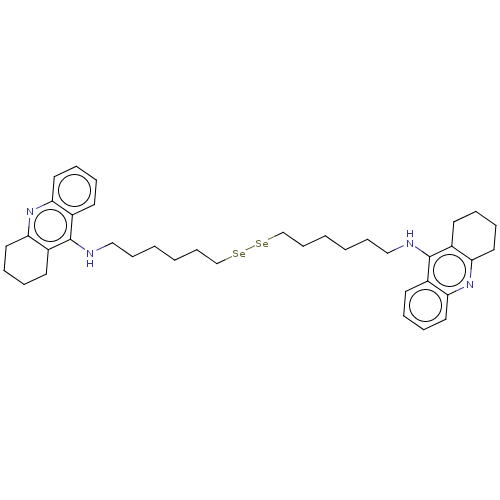 (CHEMBL4213577) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456715 (CHEMBL4213577) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456709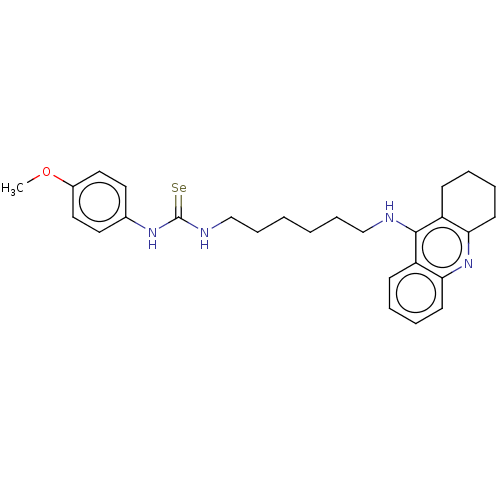 (CHEMBL4205454) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456709 (CHEMBL4205454) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456709 (CHEMBL4205454) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweav... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456709 (CHEMBL4205454) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk pl... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456707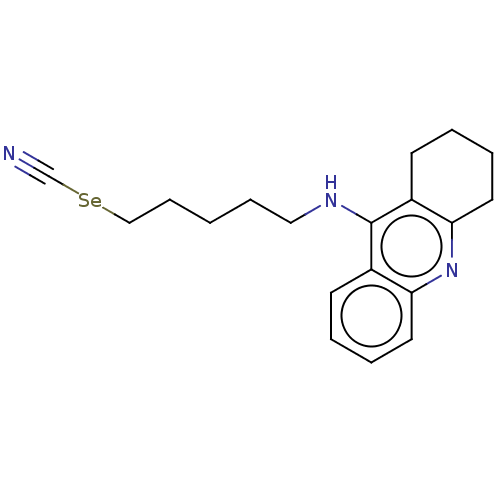 (CHEMBL4209322) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot ana... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456701 (CHEMBL4218651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456702 (CHEMBL4213042) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Bu... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456715 (CHEMBL4213577) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456704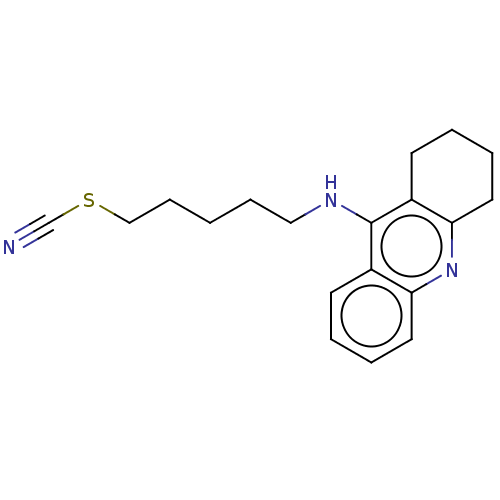 (CHEMBL4208896) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver-Burk plot... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50456704 (CHEMBL4208896) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl acetate as substrate by Lineweaver... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456706 (CHEMBL4217176) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweav... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456706 (CHEMBL4217176) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk pl... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456714 (CHEMBL4217969) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Bu... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456715 (CHEMBL4213577) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Bu... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456708 (CHEMBL4204315) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Bu... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456702 (CHEMBL4213042) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456711 (CHEMBL4208641) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20061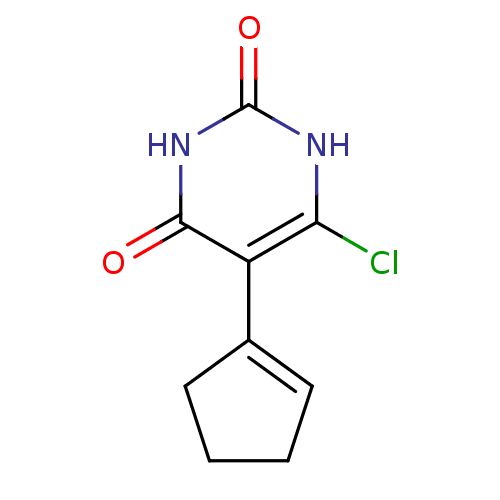 (5-Substituted-6-chlorouracil, 7a | 6-chloro-5-(cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | -39.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456707 (CHEMBL4209322) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot a... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456704 (CHEMBL4208896) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot a... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456712 (CHEMBL4204015) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456714 (CHEMBL4217969) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456703 (CHEMBL4209181) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20069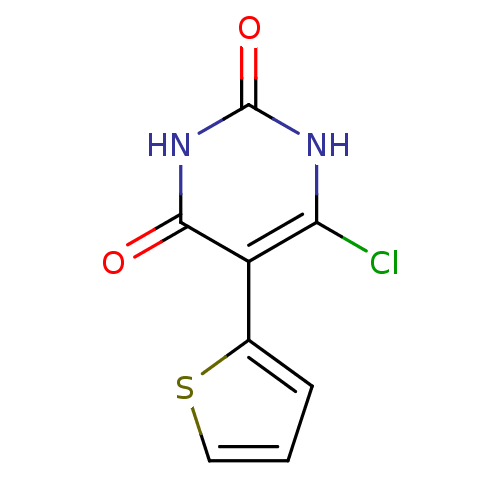 (5-Substituted-6-chlorouracil, 10e | 6-chloro-5-(th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 280 | -38.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50456712 (CHEMBL4204015) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 326 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE assessed as enzyme-substrate-inhibitor complex using p-nitrophenyl butyrate as substrate by Lineweaver-Bu... | Eur J Med Chem 138: 761-773 (2017) Article DOI: 10.1016/j.ejmech.2017.06.048 BindingDB Entry DOI: 10.7270/Q2CV4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1118 total ) | Next | Last >> |