

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | <0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | <0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM112661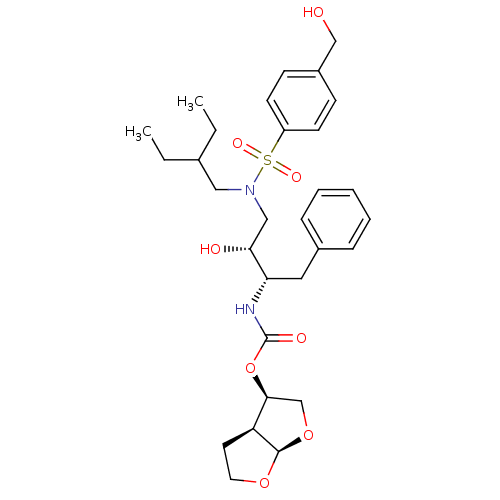 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM112656 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50545786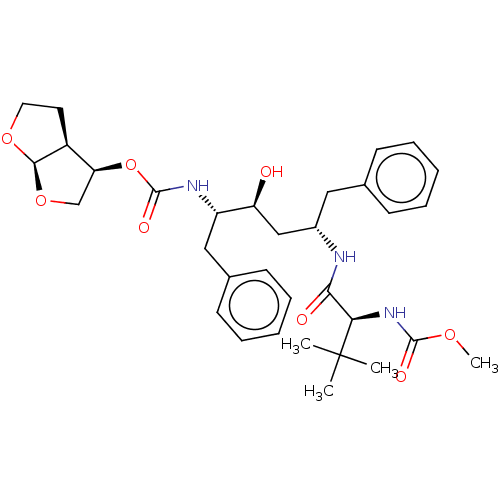 (CHEMBL4640533) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50545796 (CHEMBL4642725) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50545785 (CHEMBL4636562) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50545791 (CHEMBL4645542) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504130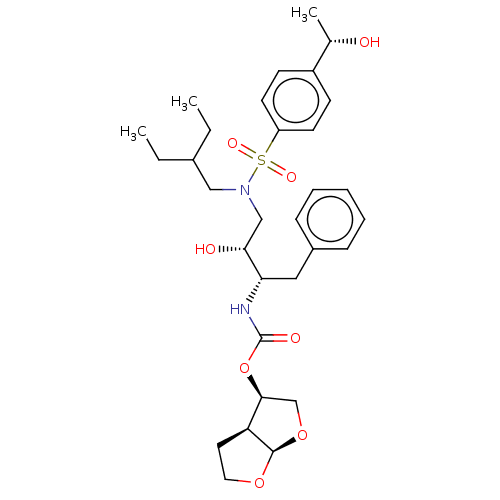 (CHEMBL4570309) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protea... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50545795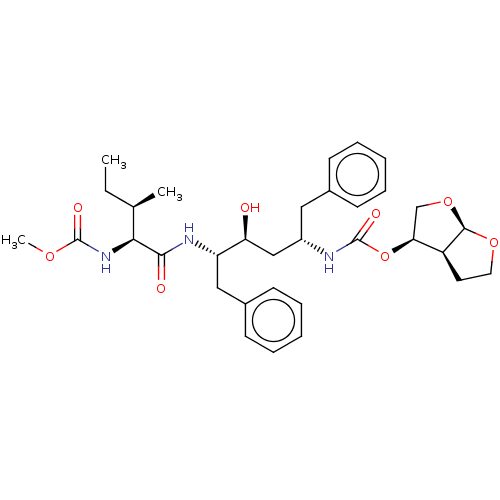 (CHEMBL4641949) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504134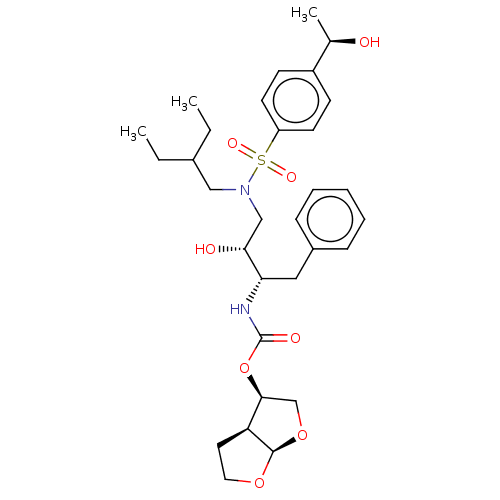 (CHEMBL4586167) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50545795 (CHEMBL4641949) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing p... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50545797 (CHEMBL4646951) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504133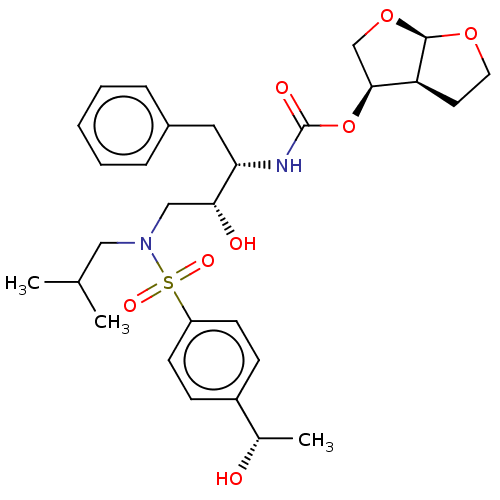 (CHEMBL4470821) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protea... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM112661 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing n... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50545794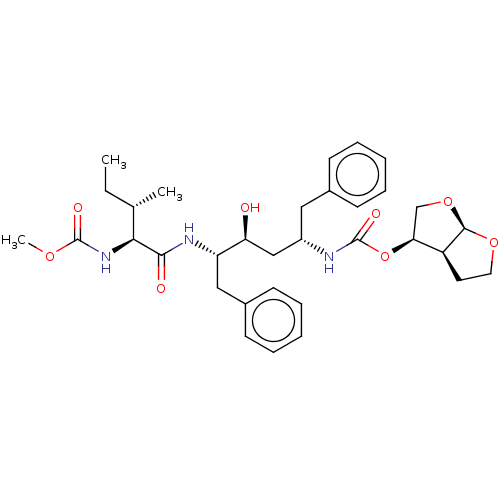 (CHEMBL4648539) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504127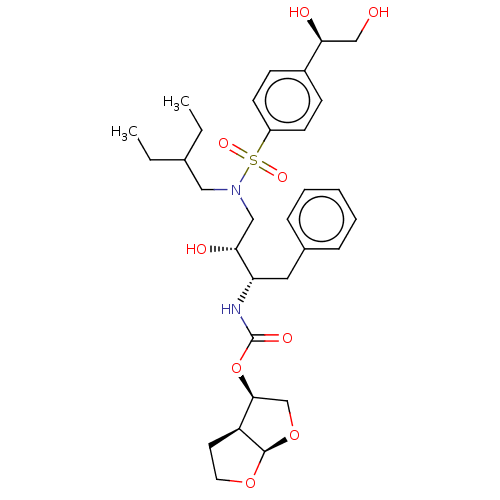 (CHEMBL4563518) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504133 (CHEMBL4470821) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing n... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing p... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50545791 (CHEMBL4645542) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protea... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50545796 (CHEMBL4642725) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protea... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50545796 (CHEMBL4642725) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing p... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50545786 (CHEMBL4640533) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing p... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50545791 (CHEMBL4645542) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing p... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50545792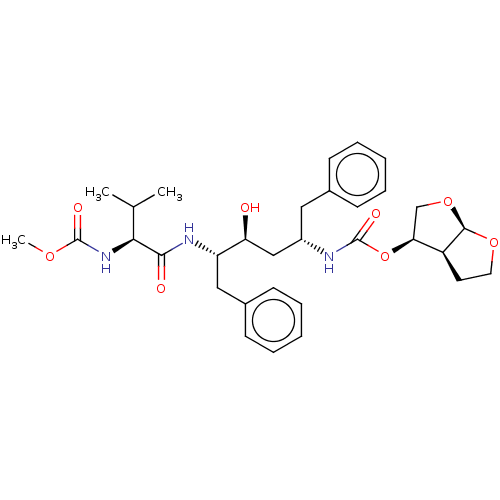 (CHEMBL4639819) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504127 (CHEMBL4563518) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing n... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing p... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing n... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM112656 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing n... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504135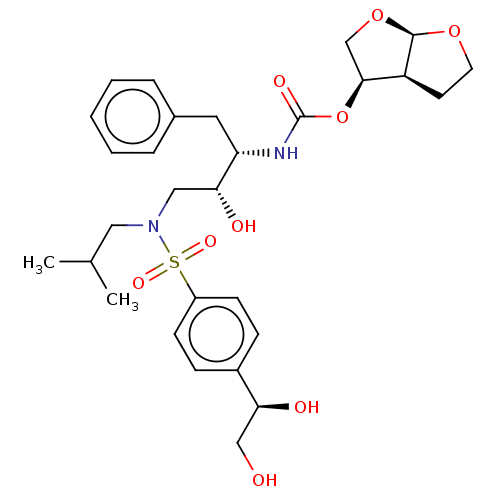 (CHEMBL4459766) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing n... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50545795 (CHEMBL4641949) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protea... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504126 (CHEMBL4472701) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing n... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504132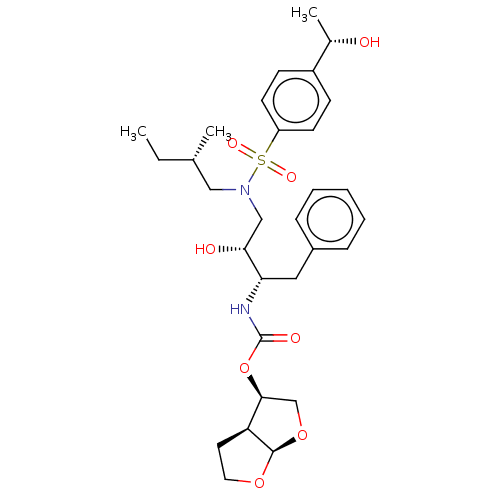 (CHEMBL4441340) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing n... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504131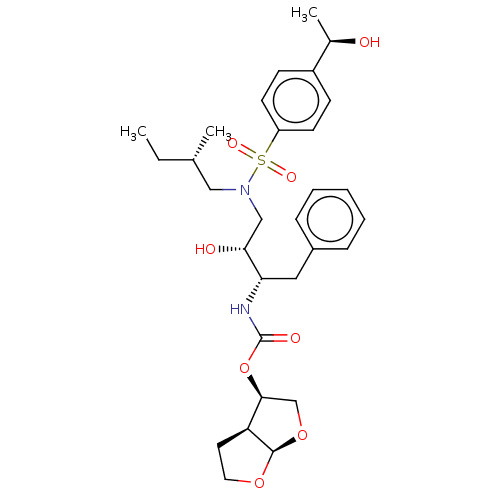 (CHEMBL4456326) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504135 (CHEMBL4459766) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504130 (CHEMBL4570309) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing n... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504134 (CHEMBL4586167) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing n... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504126 (CHEMBL4472701) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504132 (CHEMBL4441340) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504131 (CHEMBL4456326) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing n... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504128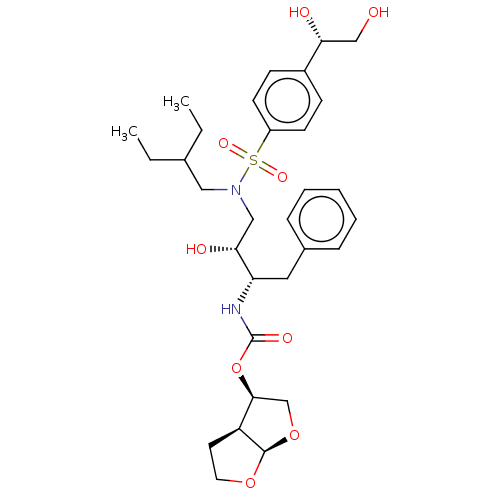 (CHEMBL4527553) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50545790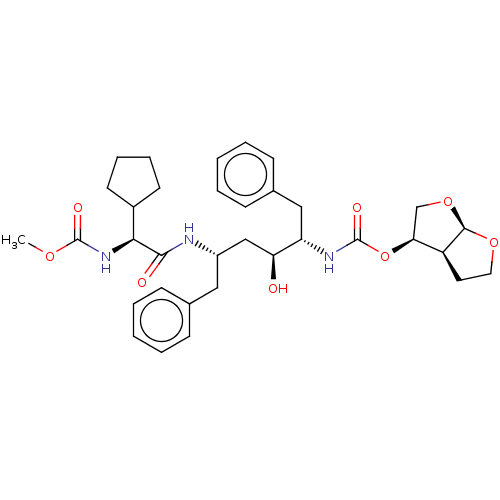 (CHEMBL4644235) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50545794 (CHEMBL4648539) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protea... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50545785 (CHEMBL4636562) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protea... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504129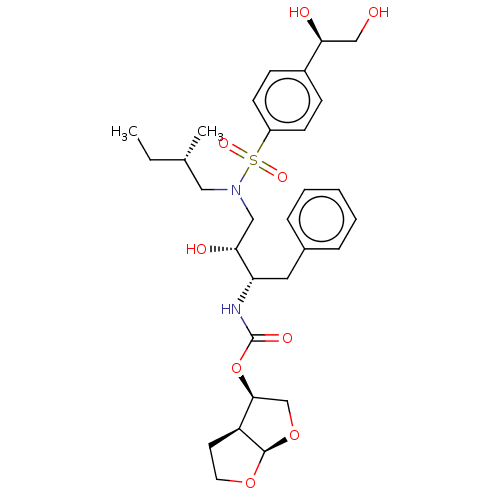 (CHEMBL4521688) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504128 (CHEMBL4527553) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I50V/A71V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing n... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50545789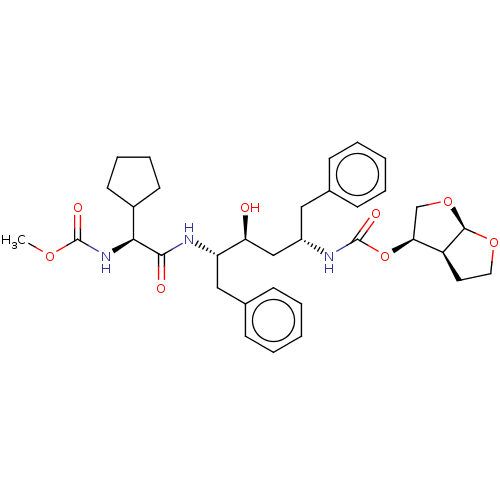 (CHEMBL4648875) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing protease cleavage ... | J Med Chem 63: 8296-8313 (2020) Article DOI: 10.1021/acs.jmedchem.0c00529 BindingDB Entry DOI: 10.7270/Q2959N46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 71 total ) | Next | Last >> |