

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50134434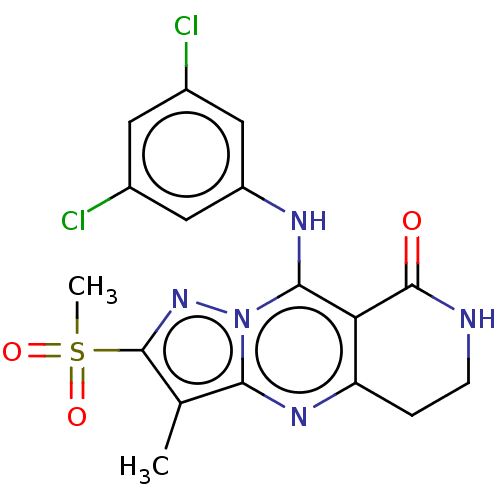 (CHEMBL3746046) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B1 expressed in insect Sf9 cells by fluorescence capillary-electrophoresis assay | Bioorg Med Chem Lett 26: 454-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.093 BindingDB Entry DOI: 10.7270/Q2X92D4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50134433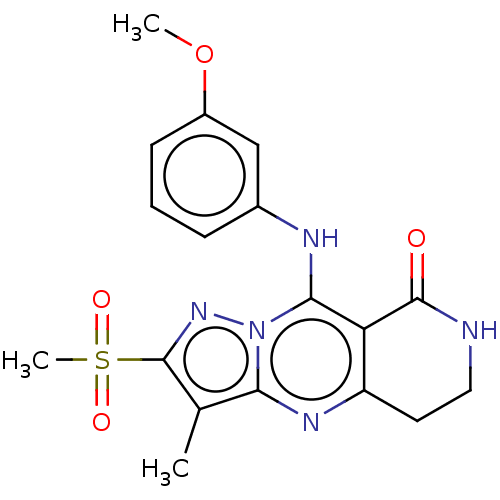 (CHEMBL3745968) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B1 expressed in insect Sf9 cells by fluorescence capillary-electrophoresis assay | Bioorg Med Chem Lett 26: 454-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.093 BindingDB Entry DOI: 10.7270/Q2X92D4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50134432 (CHEMBL3747036) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B1 expressed in insect Sf9 cells by fluorescence capillary-electrophoresis assay | Bioorg Med Chem Lett 26: 454-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.093 BindingDB Entry DOI: 10.7270/Q2X92D4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50134429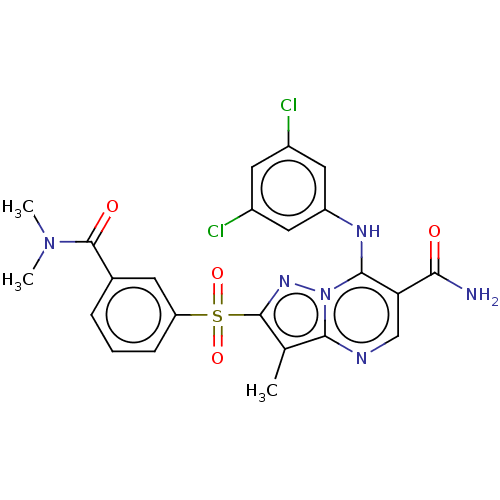 (CHEMBL3746964) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B1 expressed in insect Sf9 cells by fluorescence capillary-electrophoresis assay | Bioorg Med Chem Lett 26: 454-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.093 BindingDB Entry DOI: 10.7270/Q2X92D4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50473625 (CHEMBL69319) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma S.A. Curated by ChEMBL | Assay Description Inhibition of p38-related TNF alpha release by human monocyte cell line (THP-1) | J Med Chem 45: 2173-84 (2002) Article DOI: 10.1021/jm011132l BindingDB Entry DOI: 10.7270/Q2WW7MFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521421 (6-(2,4- dichlorophenyl)- 1-fluoro-5-[6- [(3S)-1-(3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521255 (3-(6-ethoxy-2- fluoro-3- pyridyl)-4-[6- [(3S)-1-(3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521262 (6-(6-ethoxy-2- fluoro-3- pyridyl)-5-[4- [[(3S)-1-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521384 (4-(6-ethoxy-2- fluoro-3- pyridyl)-5-[6- [(3S)-1-(3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521413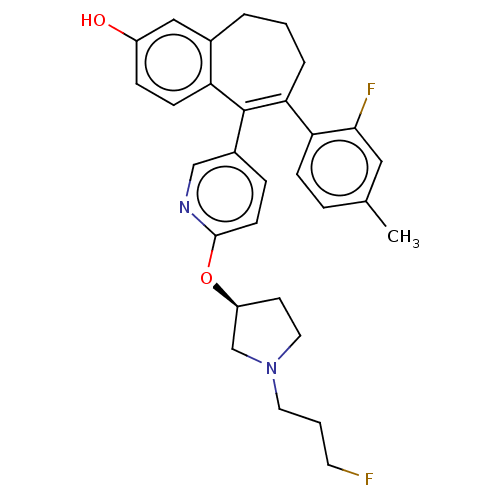 (6-(2-fluoro-4- methyl-phenyl)- 5-[6-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521382 (4-(2-chloro-4- methyl-phenyl)- 5-[6-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521428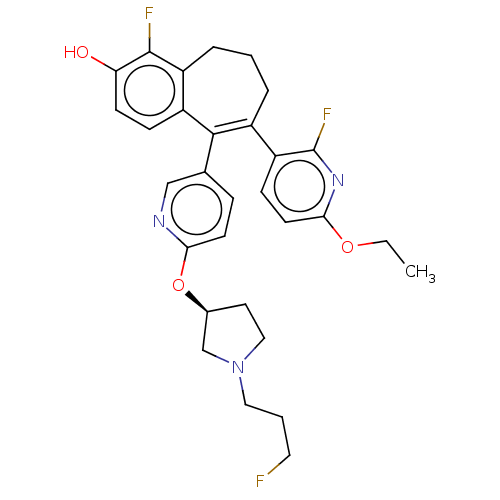 (6-(6-ethoxy-2- fluoro-3- pyridyl)-1- fluoro-5-[6- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50134428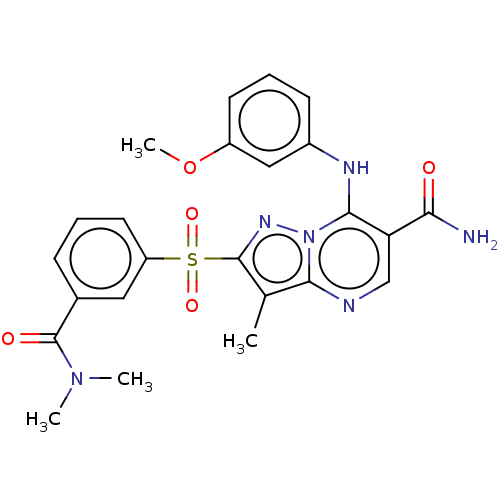 (CHEMBL3746007) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Research Center Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B1 expressed in insect Sf9 cells by fluorescence capillary-electrophoresis assay | Bioorg Med Chem Lett 26: 454-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.093 BindingDB Entry DOI: 10.7270/Q2X92D4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263706 (6-(2- chloro-4- ethoxy- phenyl)-5- [4-[(3S)-1- (3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521261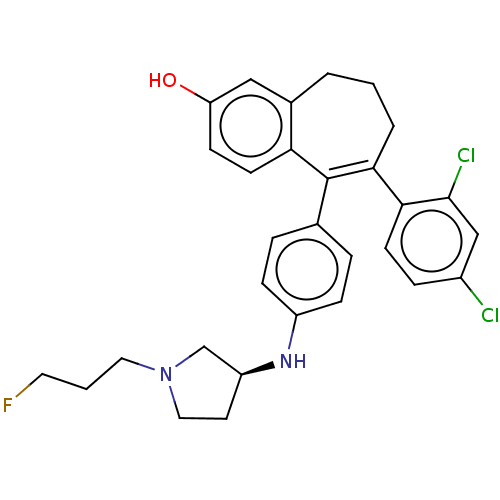 (6-(2,4- dichlorophenyl)- 5-[4-[[(3S)-1- (3- fluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50447088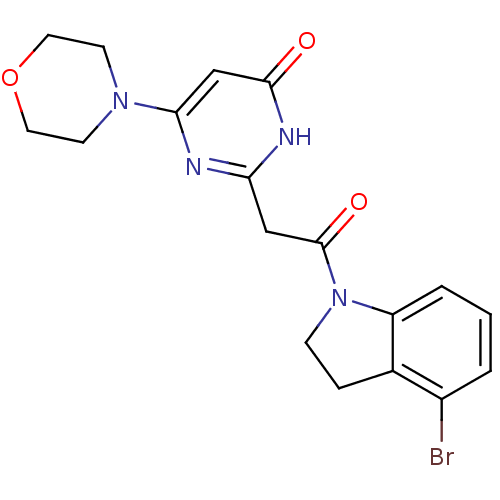 (CHEMBL3112850) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description This test is based on measuring the expression of the AKT protein phosphorylated on serine 473 (P-AKT-S473), in the PC3 human prostate carcinoma line... | US Patent US8993565 (2015) BindingDB Entry DOI: 10.7270/Q2FQ9V9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521254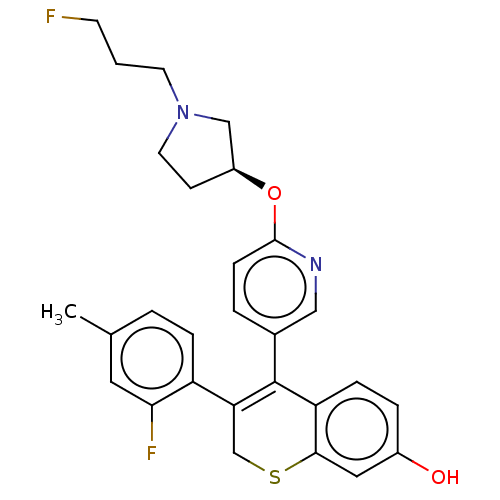 (3-(2-fluoro-4- methyl-phenyl)- 4-[6-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521417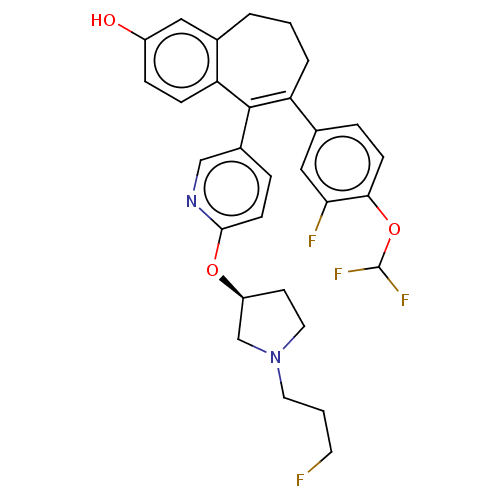 (6-[4- (difluoromethoxy)- 3-fluoro- phenyl]-5-[6- [...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521418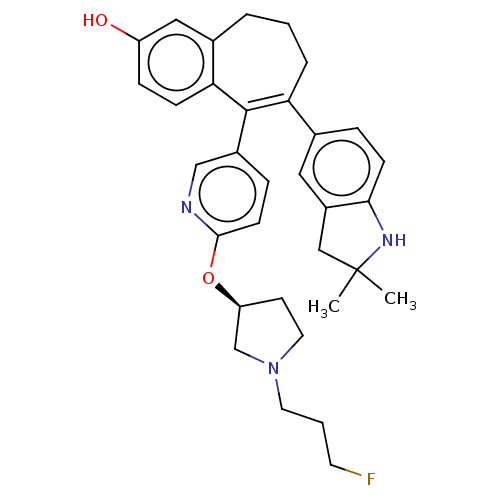 (6-(2,2- dimethylindolin- 5-yl)-5-[6-[(3S)- 1-(3- f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521425 (6-[4- (difluoromethoxy)- 3-fluoro- phenyl]-1- fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521381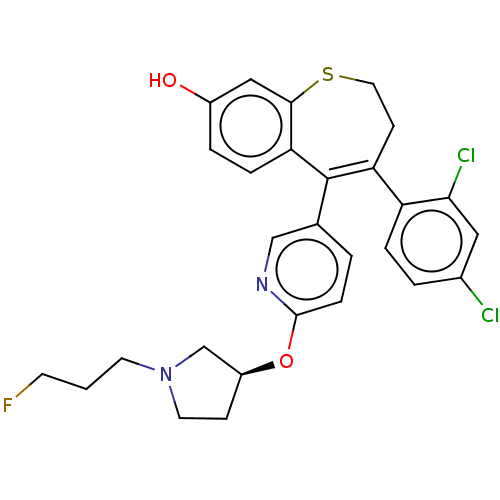 (4-(2,4- dichlorophenyl)- 5-[(6-[(3S)-1-(3- fluorop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521394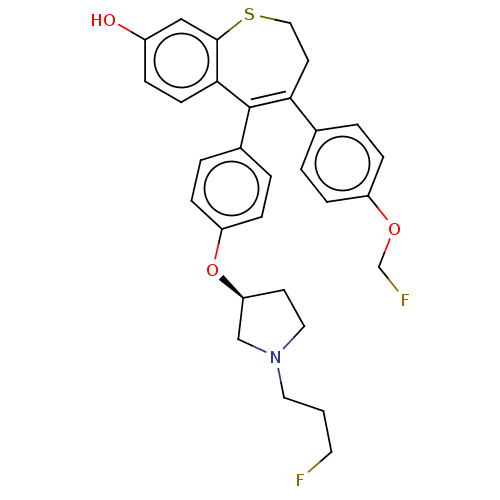 (4-[4- (fluoromethoxy) phenyl]-5-[4- [(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521360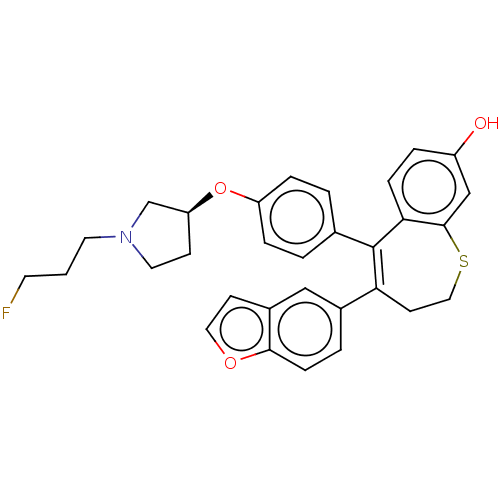 (4-(benzofuran- 5-yl)-5-[4-[(3S)- 1-(3- fluoropropy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521362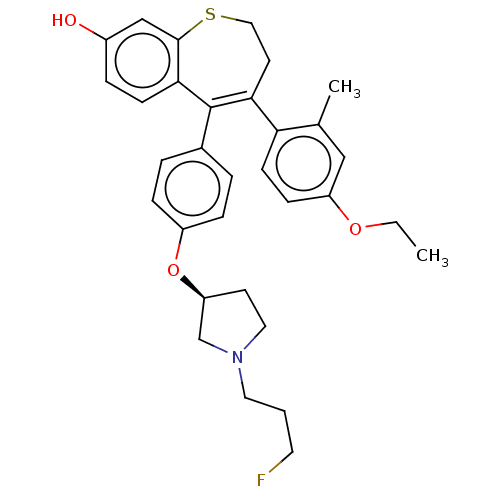 (4-(4-ethoxy-2- methyl-phenyl)- 5-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521221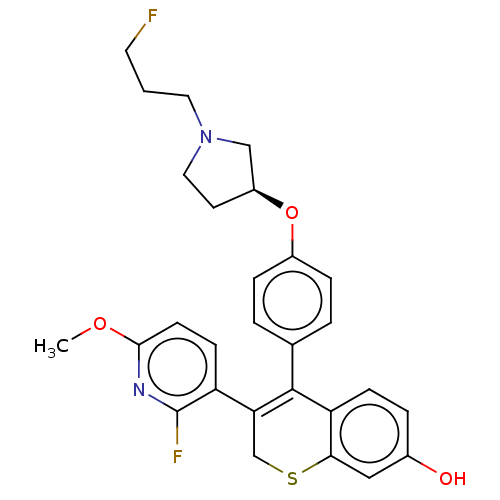 (3-(2-fluoro-6- methoxy-3- pyridyl)-4-[4- [(3S)-1-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521369 (4-(2,2-difluoro- 1,3- benzodioxol-5- yl)-5-[4-[(3S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521373 (5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521374 (4-(2,2- dimethylindolin- 5-yl)-5-[4-[(3S)- 1-(3- f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521377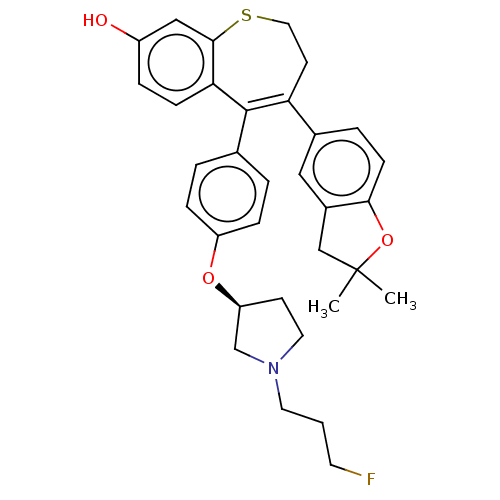 (4-(2,2-dimethyl- 3H-benzofuran- 5-yl)-5-[4-[(3S)- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521378 (5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554] (Homo sapiens (Human)) | BDBM521334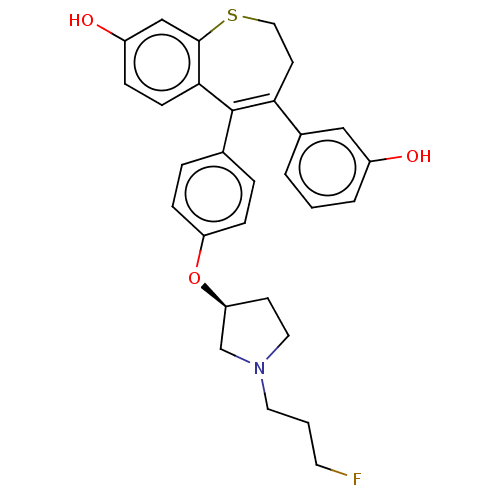 (5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263847 (6-(2,2-dimethylindolin-5-yl)-5-[4-[(3S)-1-(3-fluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263774 (US9714221, Example 108) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263775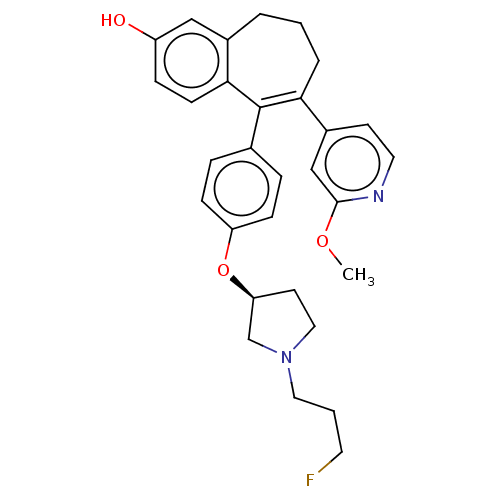 (US9714221, Example 109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263743 (5-[4-[(3S)- 1-(3- fluoropropyl)- pyrrolidin-3- yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM180381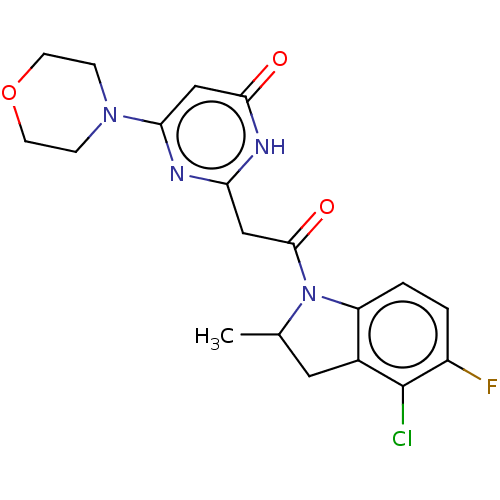 (US9133168, Example 37c | US9133168, Example 38c) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description This test is based on measurement of the expression of AKT protein phosphorylated on serine 473 (P-AKT-S473) in the PC3 human prostate carcinoma line... | US Patent US9133168 (2015) BindingDB Entry DOI: 10.7270/Q29W0D8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263748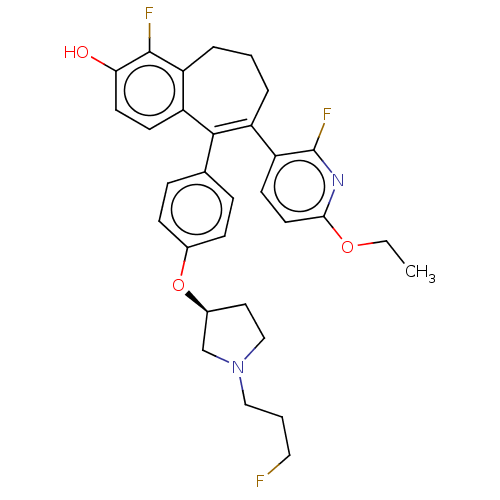 (US9714221, Example 82) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263755 (6-(2- chloro-4- methoxy- phenyl)-5- [4-[(3S)-1- (3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263764 (6-(2,2- dimethyl- 3H- benzo- furan-5-yl)-5- [4-[(3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263719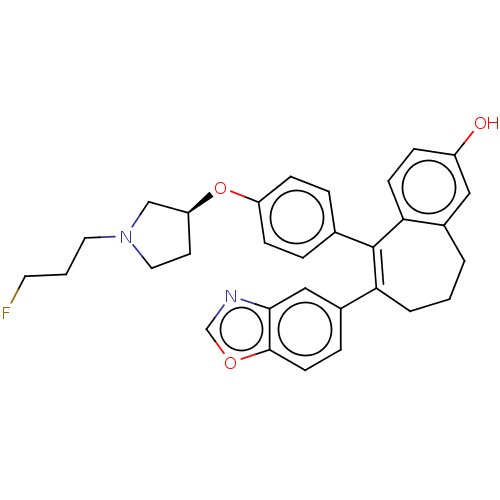 (6-(1,3- benzoxazol- 5-yl)-5-[4- [(3S)-1-(3- fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263737 (6-(6- ethoxy-2- fluoro-3- pyridyl)-5- [4-[(3S)- 1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263707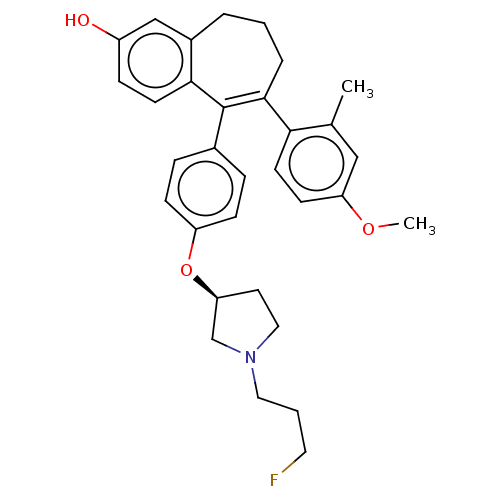 (5-[4-[(3S)- 1-(3- fluoropropyl)- pyrrolidin-3- yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263709 (6-(4- ethoxy-2- methyl- phenyl)-1- fluoro-5-[4- [(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263712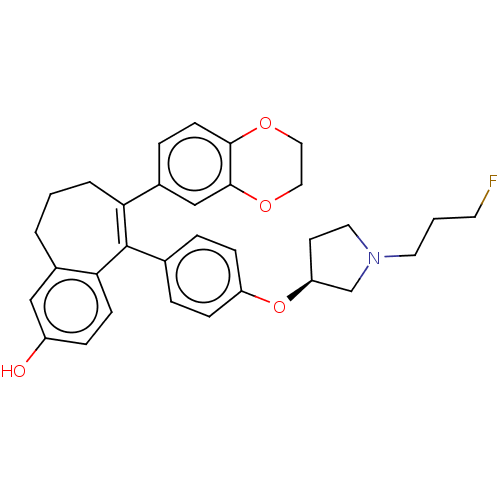 (6-(2,3- dihydro- 1,4- benzodioxin-6-yl)-5- [4-[(3S...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263900 (6-[2,3- difluoro-4- (1-piperidyl)- phenyl]-5- [4-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263911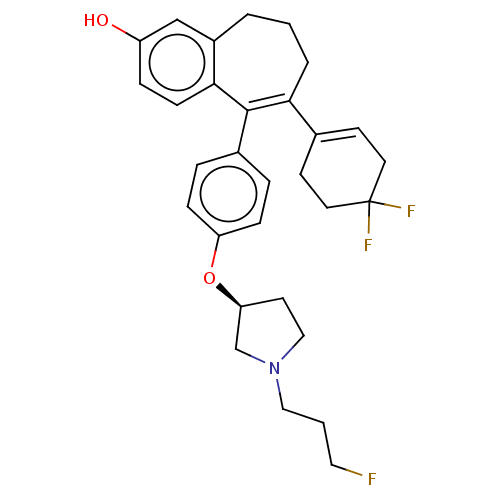 (US9714221, Example 207) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263860 (5-[4-[(3S)- 1-(3- fluoropropyl)- pyrrolidin-3- yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263892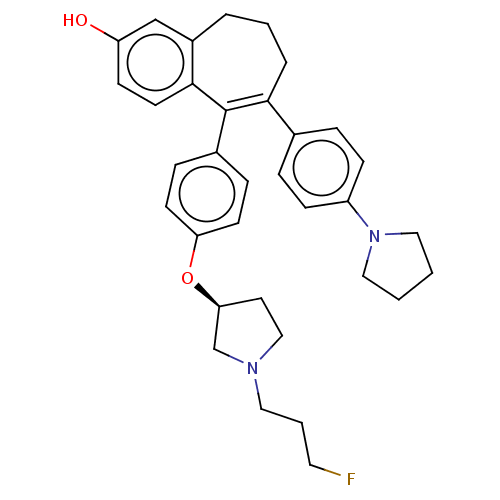 (5-[4-[(3S)- 1-(3- fluoropropyl)- pyrrolidin-3- yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM263893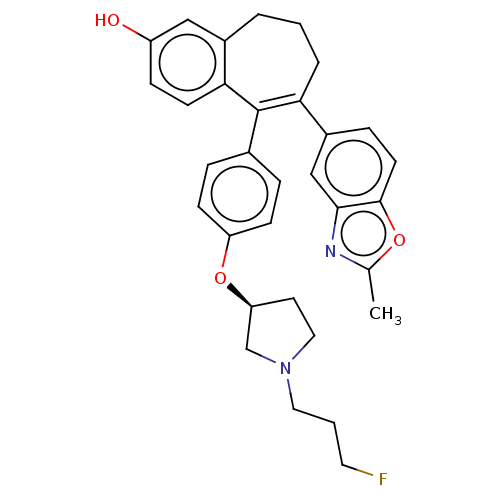 (5-[4-[(3S)- 1-(3- fluoropropyl)- pyrrolidin-3- yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
SANOFI US Patent | Assay Description It is a competition assay, where binding of a test compound to a complex comprised of (i) His6-ERα298-554 protein representing ERα ligand-b... | US Patent US9714221 (2017) BindingDB Entry DOI: 10.7270/Q2N58PB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50447088 (CHEMBL3112850) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Curated by ChEMBL | Assay Description Inhibition of N-terminal ploy-His-tagged human PI3Kbeta expressed in baculovirus-infected sf21 cells using PI(4,5)P2 as substrate after 15 mins by HT... | J Med Chem 57: 903-20 (2014) Article DOI: 10.1021/jm401642q BindingDB Entry DOI: 10.7270/Q2R212VJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2687 total ) | Next | Last >> |