 Found 437 hits with Last Name = 'lopes' and Initial = 'f'
Found 437 hits with Last Name = 'lopes' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(3) dopamine receptor
(Homo sapiens) | BDBM50610174
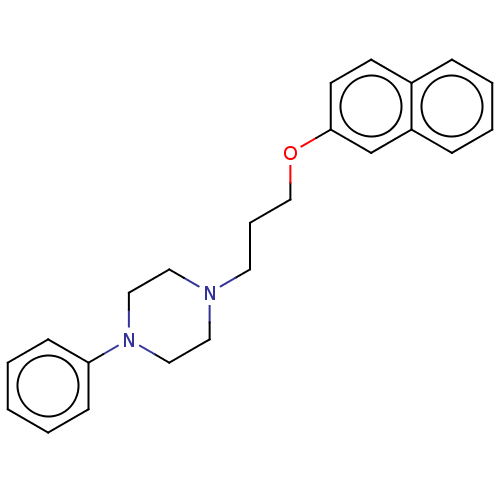
(CHEMBL4293814) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610173
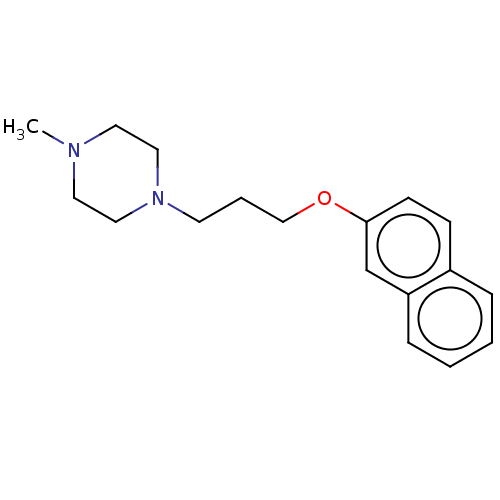
(CHEMBL5280702) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610180
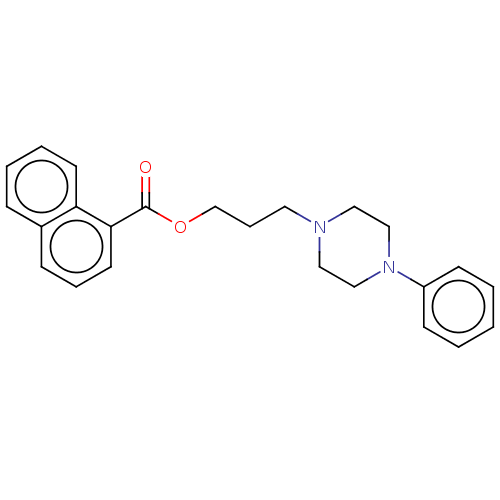
(CHEMBL5276588) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610178

(CHEMBL5289468) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610177

(CHEMBL5268329) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610182
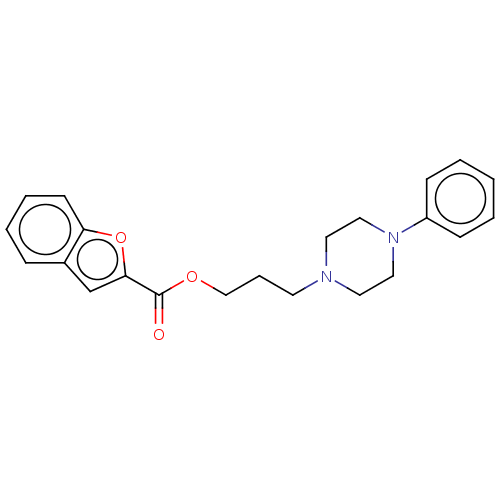
(CHEMBL5287235) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610169
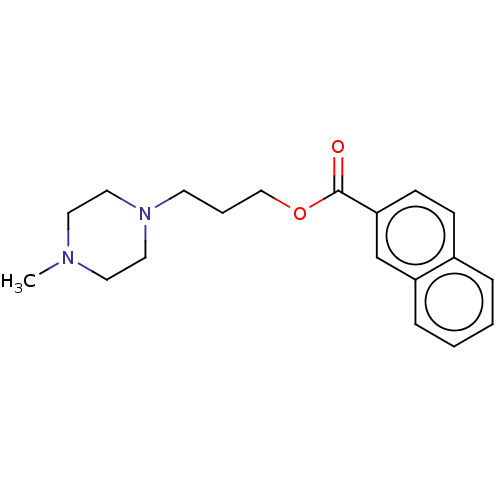
(CHEMBL5270151) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610176
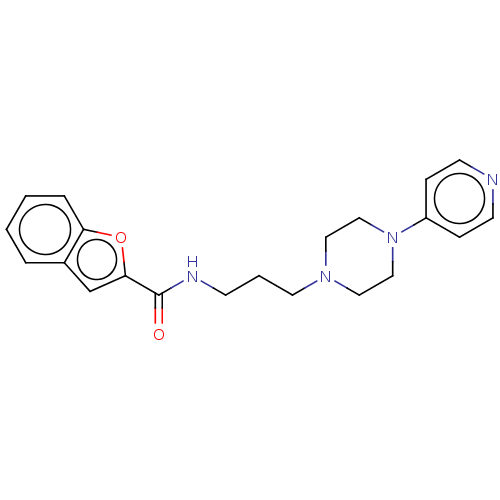
(CHEMBL5265920) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610179

(CHEMBL5282002) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610175
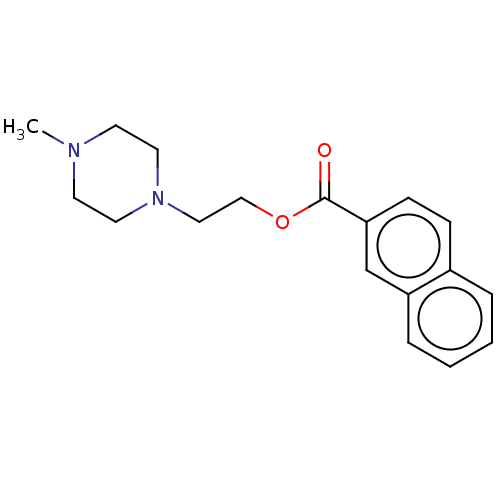
(CHEMBL5283987) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610181
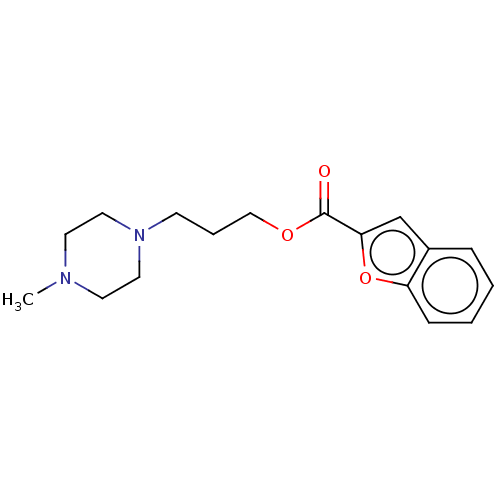
(CHEMBL5270288) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50610170

(CHEMBL5278711) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50405713
(CHEMBL4173854)Show InChI InChI=1S/C15H18ClNO3S2/c1-9(8-21)14(18)17-7-12(6-13(17)15(19)20)22-11-4-2-10(16)3-5-11/h2-5,9,12-13,21H,6-8H2,1H3,(H,19,20)/t9-,12+,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50610170

(CHEMBL5278711) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50610176

(CHEMBL5265920) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50610169

(CHEMBL5270151) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50610182

(CHEMBL5287235) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50610180

(CHEMBL5276588) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50610175

(CHEMBL5283987) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50610173

(CHEMBL5280702) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50610181

(CHEMBL5270288) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50610179

(CHEMBL5282002) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50610178

(CHEMBL5289468) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50610174

(CHEMBL4293814) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
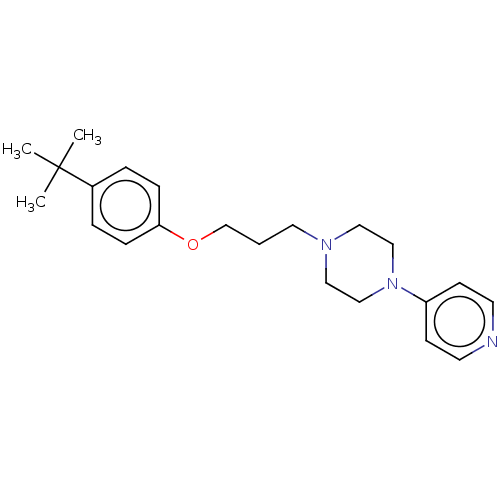
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482454
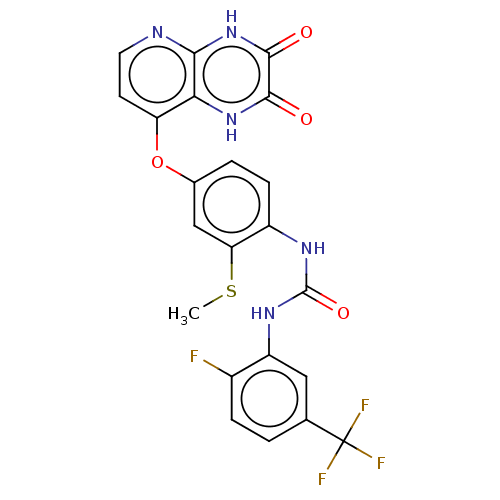
(CHEMBL1214000)Show SMILES CSc1cc(Oc2ccnc3[nH]c(=O)c(=O)[nH]c23)ccc1NC(=O)Nc1cc(ccc1F)C(F)(F)F Show InChI InChI=1S/C22H15F4N5O4S/c1-36-16-9-11(35-15-6-7-27-18-17(15)30-19(32)20(33)31-18)3-5-13(16)28-21(34)29-14-8-10(22(24,25)26)2-4-12(14)23/h2-9H,1H3,(H,30,32)(H,27,31,33)(H2,28,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482455
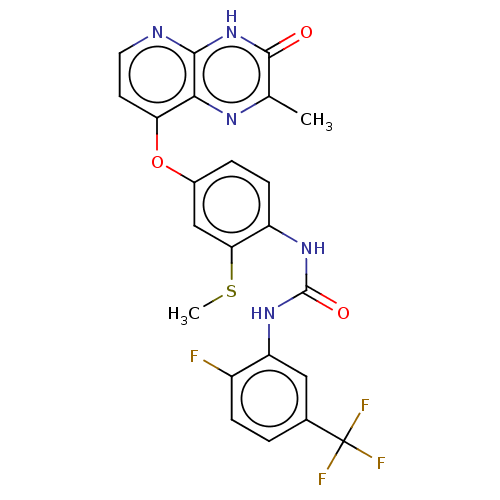
(CHEMBL1214001)Show SMILES CSc1cc(Oc2ccnc3[nH]c(=O)c(C)nc23)ccc1NC(=O)Nc1cc(ccc1F)C(F)(F)F Show InChI InChI=1S/C23H17F4N5O3S/c1-11-21(33)32-20-19(29-11)17(7-8-28-20)35-13-4-6-15(18(10-13)36-2)30-22(34)31-16-9-12(23(25,26)27)3-5-14(16)24/h3-10H,1-2H3,(H,28,32,33)(H2,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482509
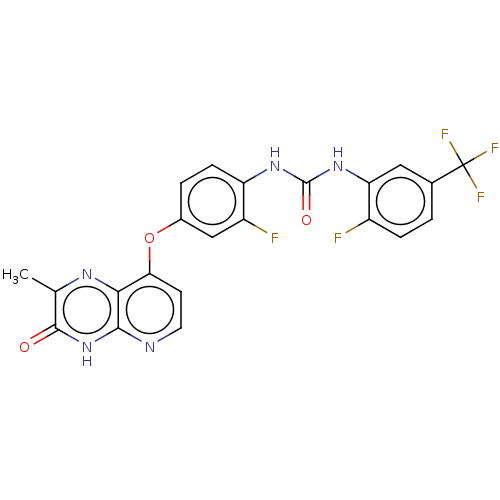
(CHEMBL1214614)Show SMILES Cc1nc2c(Oc3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)c(F)c3)ccnc2[nH]c1=O Show InChI InChI=1S/C22H14F5N5O3/c1-10-20(33)32-19-18(29-10)17(6-7-28-19)35-12-3-5-15(14(24)9-12)30-21(34)31-16-8-11(22(25,26)27)2-4-13(16)23/h2-9H,1H3,(H,28,32,33)(H2,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482510
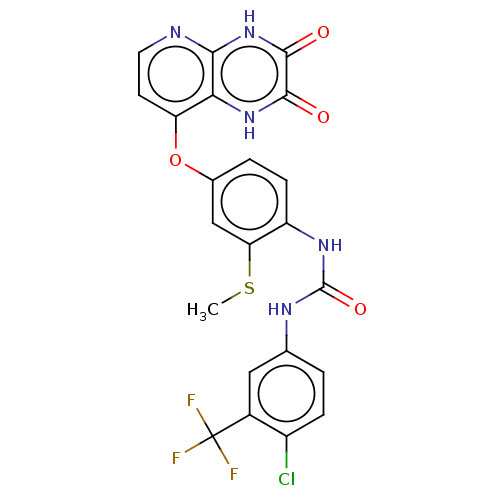
(CHEMBL1213998)Show SMILES CSc1cc(Oc2ccnc3[nH]c(=O)c(=O)[nH]c23)ccc1NC(=O)Nc1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C22H15ClF3N5O4S/c1-36-16-9-11(35-15-6-7-27-18-17(15)30-19(32)20(33)31-18)3-5-14(16)29-21(34)28-10-2-4-13(23)12(8-10)22(24,25)26/h2-9H,1H3,(H,30,32)(H,27,31,33)(H2,28,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482489
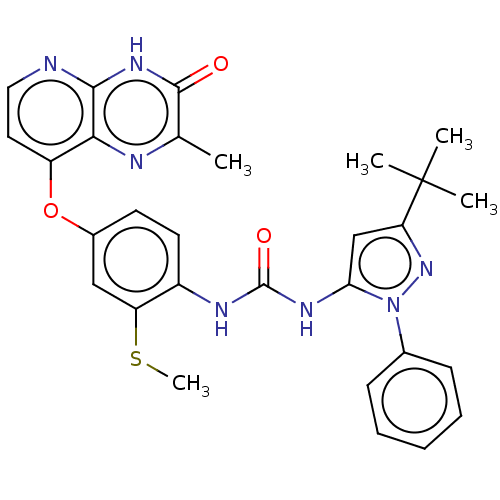
(CHEMBL1214055)Show SMILES CSc1cc(Oc2ccnc3[nH]c(=O)c(C)nc23)ccc1NC(=O)Nc1cc(nn1-c1ccccc1)C(C)(C)C Show InChI InChI=1S/C29H29N7O3S/c1-17-27(37)34-26-25(31-17)21(13-14-30-26)39-19-11-12-20(22(15-19)40-5)32-28(38)33-24-16-23(29(2,3)4)35-36(24)18-9-7-6-8-10-18/h6-16H,1-5H3,(H,30,34,37)(H2,32,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant-mediated ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482490
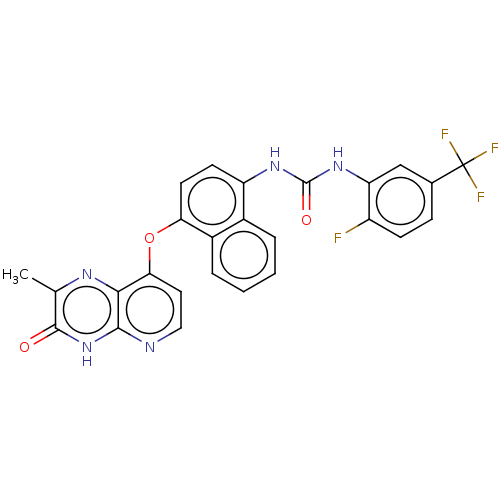
(CHEMBL1214058)Show SMILES Cc1nc2c(Oc3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)c4ccccc34)ccnc2[nH]c1=O Show InChI InChI=1S/C26H17F4N5O3/c1-13-24(36)35-23-22(32-13)21(10-11-31-23)38-20-9-8-18(15-4-2-3-5-16(15)20)33-25(37)34-19-12-14(26(28,29)30)6-7-17(19)27/h2-12H,1H3,(H,31,35,36)(H2,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482482
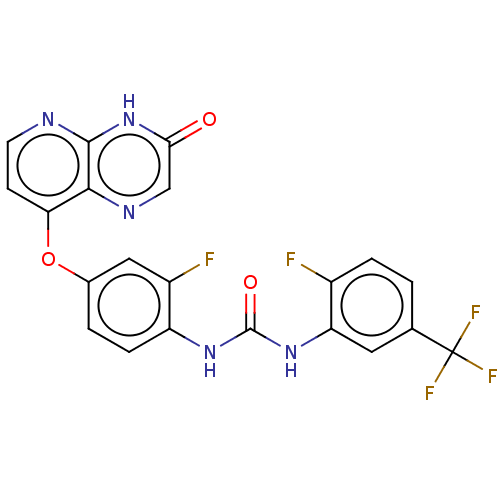
(CHEMBL1214612)Show SMILES Fc1ccc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)cnc23)cc1F)C(F)(F)F Show InChI InChI=1S/C21H12F5N5O3/c22-12-3-1-10(21(24,25)26)7-15(12)30-20(33)29-14-4-2-11(8-13(14)23)34-16-5-6-27-19-18(16)28-9-17(32)31-19/h1-9H,(H,27,31,32)(H2,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482488
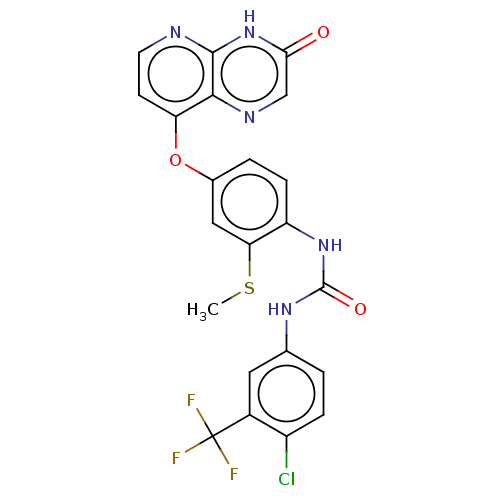
(CHEMBL1213952)Show SMILES CSc1cc(Oc2ccnc3[nH]c(=O)cnc23)ccc1NC(=O)Nc1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C22H15ClF3N5O3S/c1-35-17-9-12(34-16-6-7-27-20-19(16)28-10-18(32)31-20)3-5-15(17)30-21(33)29-11-2-4-14(23)13(8-11)22(24,25)26/h2-10H,1H3,(H,27,31,32)(H2,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482453

(CHEMBL1213999)Show SMILES CSc1cc(Oc2ccnc3[nH]c(=O)cnc23)ccc1NC(=O)Nc1cc(ccc1F)C(F)(F)F Show InChI InChI=1S/C22H15F4N5O3S/c1-35-17-9-12(34-16-6-7-27-20-19(16)28-10-18(32)31-20)3-5-14(17)29-21(33)30-15-8-11(22(24,25)26)2-4-13(15)23/h2-10H,1H3,(H,27,31,32)(H2,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482512
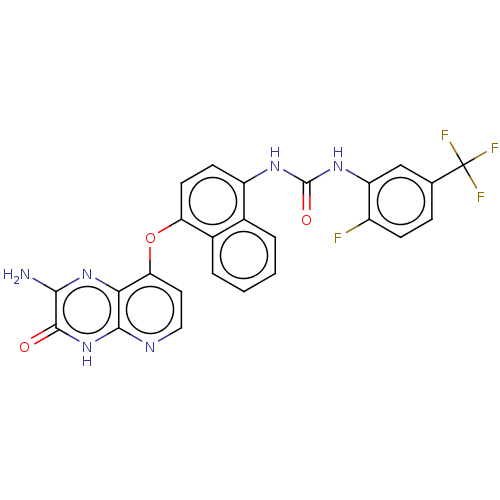
(CHEMBL1214129)Show SMILES Nc1nc2c(Oc3ccc(NC(=O)Nc4cc(ccc4F)C(F)(F)F)c4ccccc34)ccnc2[nH]c1=O Show InChI InChI=1S/C25H16F4N6O3/c26-15-6-5-12(25(27,28)29)11-17(15)33-24(37)32-16-7-8-18(14-4-2-1-3-13(14)16)38-19-9-10-31-22-20(19)34-21(30)23(36)35-22/h1-11H,(H2,30,34)(H,31,35,36)(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482514
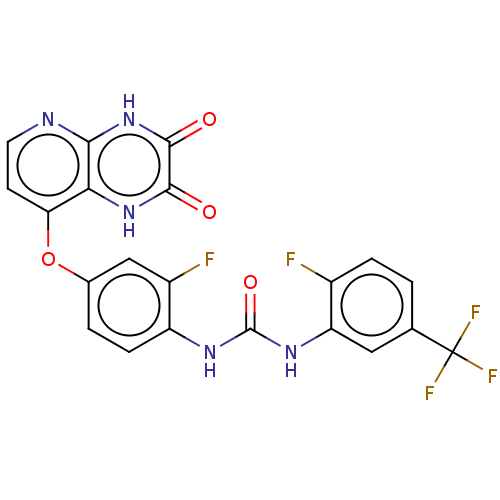
(CHEMBL1213691)Show SMILES Fc1ccc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)c(=O)[nH]c23)cc1F)C(F)(F)F Show InChI InChI=1S/C21H12F5N5O4/c22-11-3-1-9(21(24,25)26)7-14(11)29-20(34)28-13-4-2-10(8-12(13)23)35-15-5-6-27-17-16(15)30-18(32)19(33)31-17/h1-8H,(H,30,32)(H,27,31,33)(H2,28,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482511
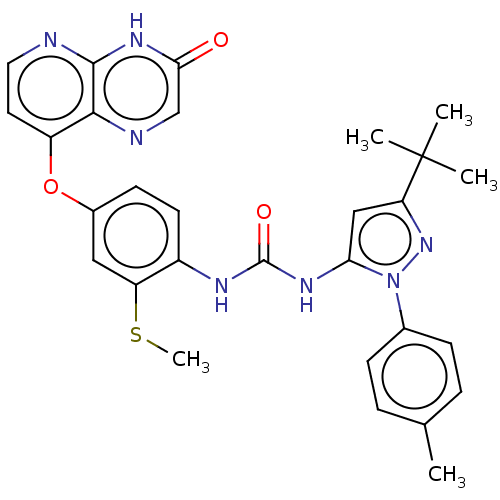
(CHEMBL1214056)Show SMILES CSc1cc(Oc2ccnc3[nH]c(=O)cnc23)ccc1NC(=O)Nc1cc(nn1-c1ccc(C)cc1)C(C)(C)C Show InChI InChI=1S/C29H29N7O3S/c1-17-6-8-18(9-7-17)36-24(15-23(35-36)29(2,3)4)33-28(38)32-20-11-10-19(14-22(20)40-5)39-21-12-13-30-27-26(21)31-16-25(37)34-27/h6-16H,1-5H3,(H,30,34,37)(H2,32,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant-mediated ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM142606
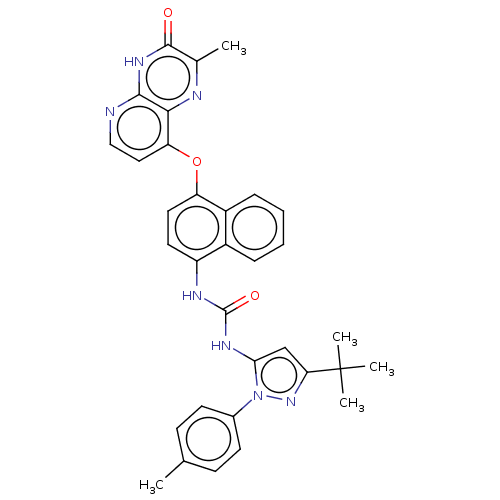
(US8933228, 8)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)c(C)nc23)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C33H31N7O3/c1-19-10-12-21(13-11-19)40-28(18-27(39-40)33(3,4)5)37-32(42)36-24-14-15-25(23-9-7-6-8-22(23)24)43-26-16-17-34-30-29(26)35-20(2)31(41)38-30/h6-18H,1-5H3,(H,34,38,41)(H2,36,37,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant-mediated ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482449
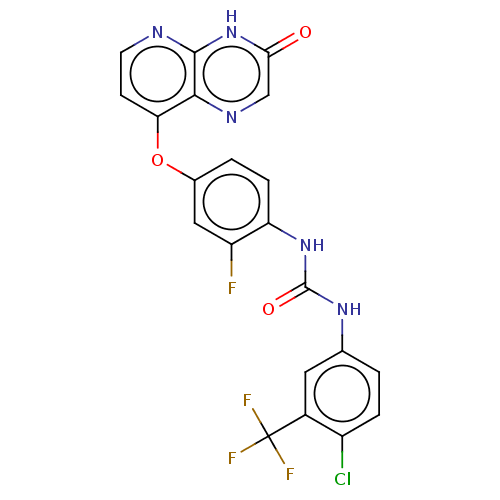
(CHEMBL1213824)Show SMILES Fc1cc(Oc2ccnc3[nH]c(=O)cnc23)ccc1NC(=O)Nc1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C21H12ClF4N5O3/c22-13-3-1-10(7-12(13)21(24,25)26)29-20(33)30-15-4-2-11(8-14(15)23)34-16-5-6-27-19-18(16)28-9-17(32)31-19/h1-9H,(H,27,31,32)(H2,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
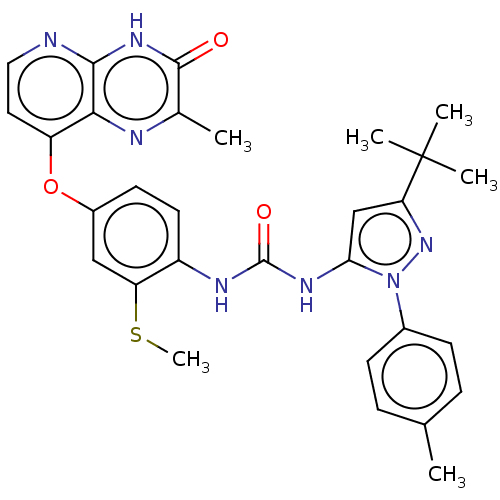
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482456
(CHEMBL1214057)Show SMILES CSc1cc(Oc2ccnc3[nH]c(=O)c(C)nc23)ccc1NC(=O)Nc1cc(nn1-c1ccc(C)cc1)C(C)(C)C Show InChI InChI=1S/C30H31N7O3S/c1-17-7-9-19(10-8-17)37-25(16-24(36-37)30(3,4)5)34-29(39)33-21-12-11-20(15-23(21)41-6)40-22-13-14-31-27-26(22)32-18(2)28(38)35-27/h7-16H,1-6H3,(H,31,35,38)(H2,33,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant-mediated ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
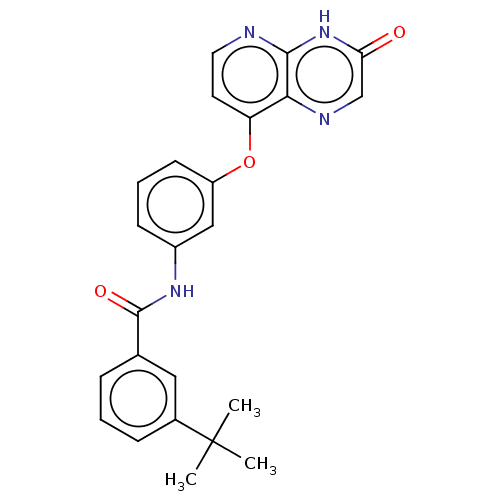
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482508
(CHEMBL1214617)Show SMILES CC(C)(C)c1cccc(c1)C(=O)Nc1cccc(Oc2ccnc3[nH]c(=O)cnc23)c1 Show InChI InChI=1S/C24H22N4O3/c1-24(2,3)16-7-4-6-15(12-16)23(30)27-17-8-5-9-18(13-17)31-19-10-11-25-22-21(19)26-14-20(29)28-22/h4-14H,1-3H3,(H,27,30)(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482491
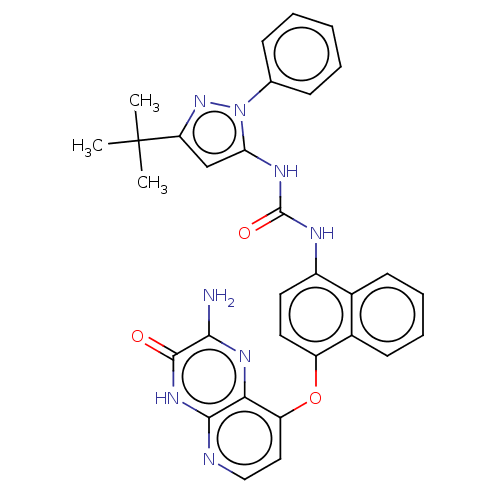
(CHEMBL1214133)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)c(N)nc34)c3ccccc23)n(n1)-c1ccccc1 Show InChI InChI=1S/C31H28N8O3/c1-31(2,3)24-17-25(39(38-24)18-9-5-4-6-10-18)35-30(41)34-21-13-14-22(20-12-8-7-11-19(20)21)42-23-15-16-33-28-26(23)36-27(32)29(40)37-28/h4-17H,1-3H3,(H2,32,36)(H,33,37,40)(H2,34,35,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant-mediated ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482492
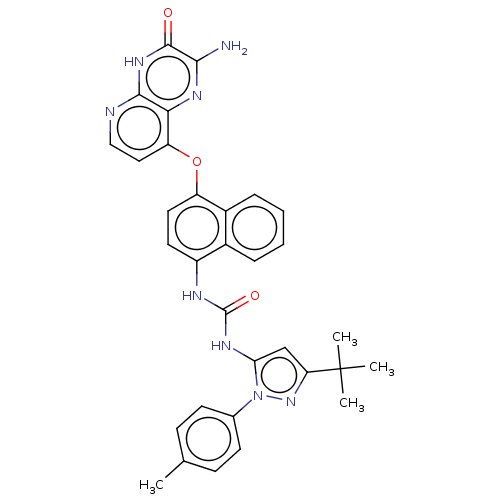
(CHEMBL1214136)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)c(N)nc23)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C32H30N8O3/c1-18-9-11-19(12-10-18)40-26(17-25(39-40)32(2,3)4)36-31(42)35-22-13-14-23(21-8-6-5-7-20(21)22)43-24-15-16-34-29-27(24)37-28(33)30(41)38-29/h5-17H,1-4H3,(H2,33,37)(H,34,38,41)(H2,35,36,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant-mediated ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM142603
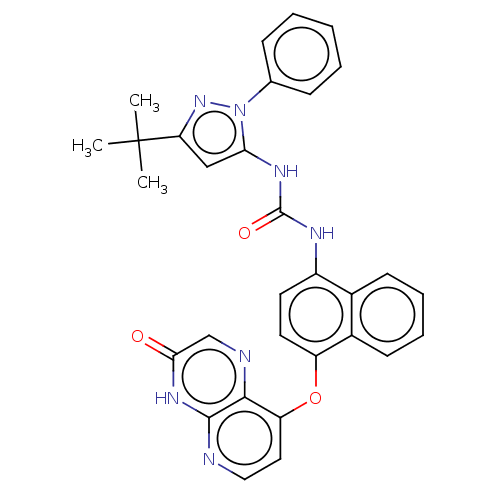
(US8933228, 5)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Oc3ccnc4[nH]c(=O)cnc34)c3ccccc23)n(n1)-c1ccccc1 Show InChI InChI=1S/C31H27N7O3/c1-31(2,3)25-17-26(38(37-25)19-9-5-4-6-10-19)35-30(40)34-22-13-14-23(21-12-8-7-11-20(21)22)41-24-15-16-32-29-28(24)33-18-27(39)36-29/h4-18H,1-3H3,(H,32,36,39)(H2,34,35,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant-mediated ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM142605
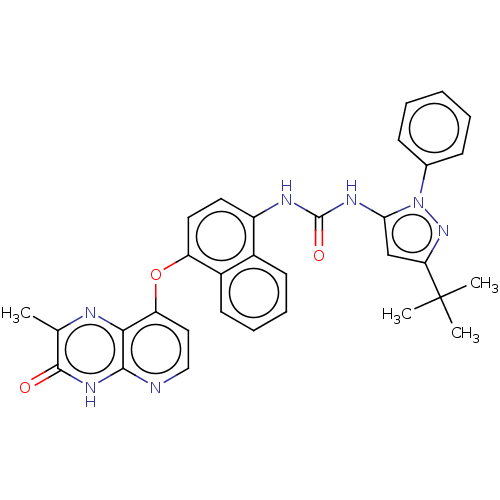
(US8933228, 7)Show SMILES Cc1nc2c(Oc3ccc(NC(=O)Nc4cc(nn4-c4ccccc4)C(C)(C)C)c4ccccc34)ccnc2[nH]c1=O Show InChI InChI=1S/C32H29N7O3/c1-19-30(40)37-29-28(34-19)25(16-17-33-29)42-24-15-14-23(21-12-8-9-13-22(21)24)35-31(41)36-27-18-26(32(2,3)4)38-39(27)20-10-6-5-7-11-20/h5-18H,1-4H3,(H,33,37,40)(H2,35,36,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant-mediated ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482476
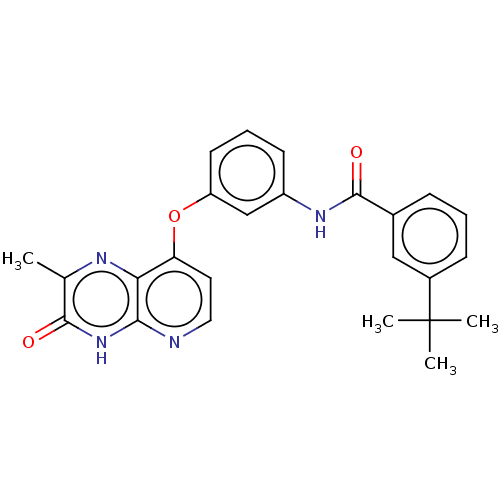
(CHEMBL1214619)Show SMILES Cc1nc2c(Oc3cccc(NC(=O)c4cccc(c4)C(C)(C)C)c3)ccnc2[nH]c1=O Show InChI InChI=1S/C25H24N4O3/c1-15-23(30)29-22-21(27-15)20(11-12-26-22)32-19-10-6-9-18(14-19)28-24(31)16-7-5-8-17(13-16)25(2,3)4/h5-14H,1-4H3,(H,28,31)(H,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM142604
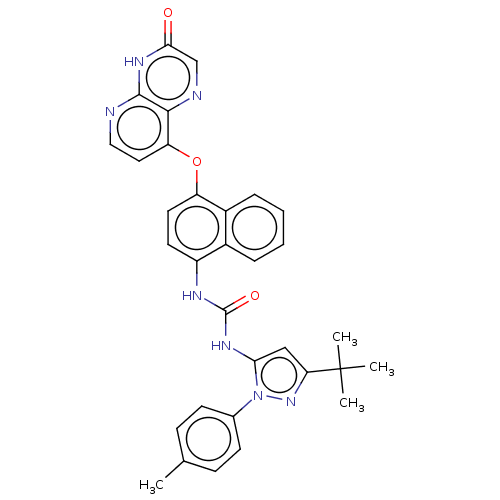
(US8933228, 6)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)cnc23)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C32H29N7O3/c1-19-9-11-20(12-10-19)39-27(17-26(38-39)32(2,3)4)36-31(41)35-23-13-14-24(22-8-6-5-7-21(22)23)42-25-15-16-33-30-29(25)34-18-28(40)37-30/h5-18H,1-4H3,(H,33,37,40)(H2,35,36,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant-mediated ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50482487
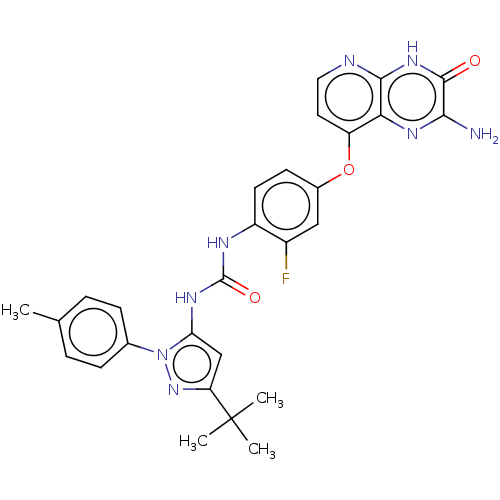
(CHEMBL1213886)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(Oc2ccnc3[nH]c(=O)c(N)nc23)cc1F)C(C)(C)C Show InChI InChI=1S/C28H27FN8O3/c1-15-5-7-16(8-6-15)37-22(14-21(36-37)28(2,3)4)33-27(39)32-19-10-9-17(13-18(19)29)40-20-11-12-31-25-23(20)34-24(30)26(38)35-25/h5-14H,1-4H3,(H2,30,34)(H,31,35,38)(H2,32,33,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant-mediated ERK phosphorylation in human WM266.4 cells |
J Med Chem 53: 5639-55 (2010)
Article DOI: 10.1021/jm100383b
BindingDB Entry DOI: 10.7270/Q22V2JZS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data