

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50427703 (CHEMBL2324220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Curated by ChEMBL | Assay Description Inhibition of human recombinant IDE expressed in CHO cells in presence of [125I]-insulin by HTRF assay | Eur J Med Chem 179: 557-566 (2019) Article DOI: 10.1016/j.ejmech.2019.06.057 BindingDB Entry DOI: 10.7270/Q2XS5ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174595 (US9102670, 14a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174368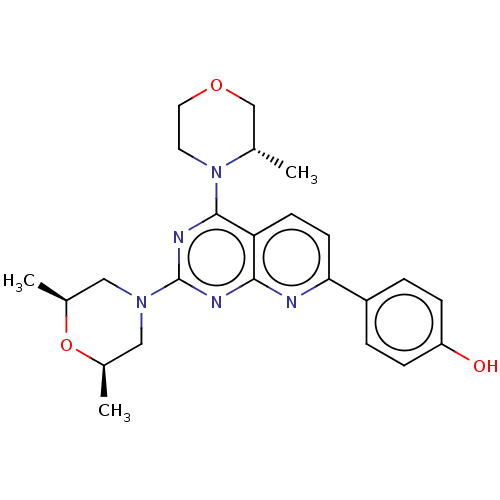 (US9102670, 1cg) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174414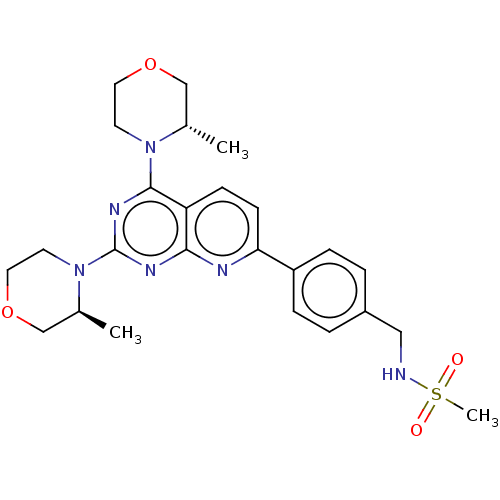 (US9102670, 1du) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50382480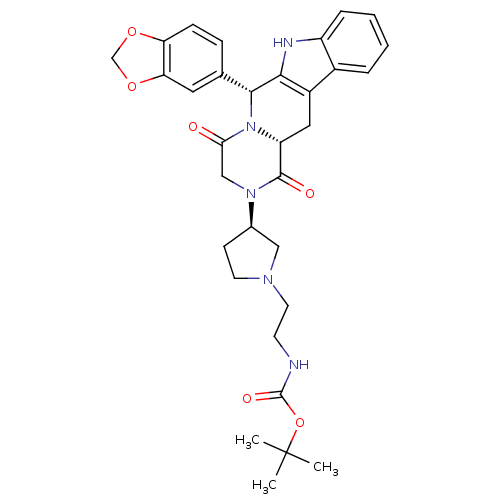 (CHEMBL2022215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of bovine PDE5 after 24 hrs by time-resolved fluorescence resonance energy transfer assay | J Med Chem 55: 1274-86 (2012) Article DOI: 10.1021/jm201422e BindingDB Entry DOI: 10.7270/Q2WW7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of human NEP-mediated amyloid beta hydrolysis | Eur J Med Chem 79: 184-93 (2014) Article DOI: 10.1016/j.ejmech.2014.04.009 BindingDB Entry DOI: 10.7270/Q27H1M34 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50382484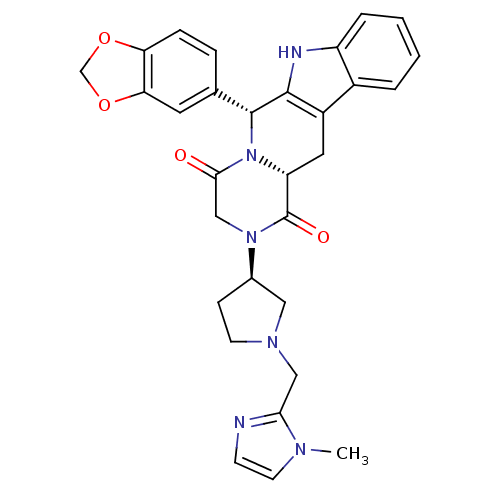 (CHEMBL2022213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of bovine PDE5 after 24 hrs by time-resolved fluorescence resonance energy transfer assay | J Med Chem 55: 1274-86 (2012) Article DOI: 10.1021/jm201422e BindingDB Entry DOI: 10.7270/Q2WW7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174467 (US9102670, 4a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174288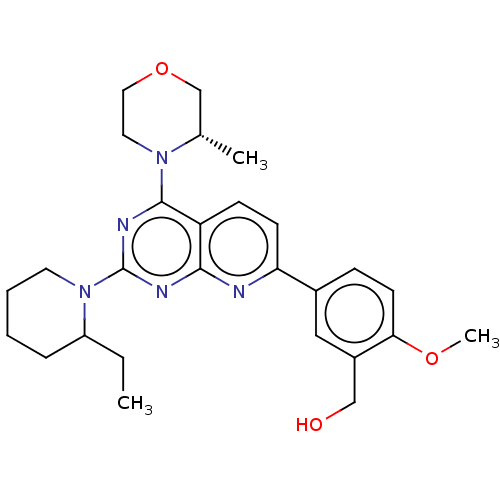 (US9102670, 1c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description For mTOR enzyme activity assays, mTOR protein was isolated from HeLa cell cytoplasmic extract by immunoprecipitation, and activity determined essenti... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174287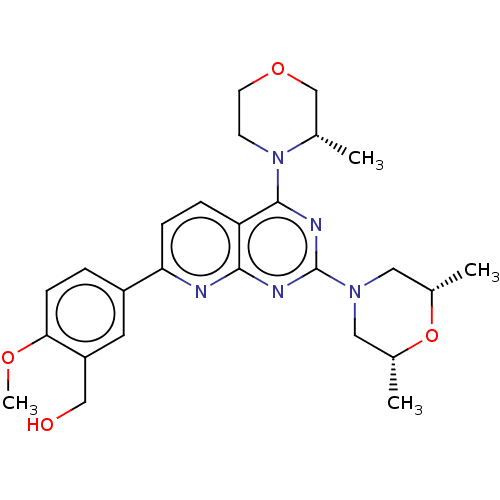 (US9102670, 1b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.85 | n/a | n/a | n/a | n/a | n/a | n/a |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description For mTOR enzyme activity assays, mTOR protein was isolated from HeLa cell cytoplasmic extract by immunoprecipitation, and activity determined essenti... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174471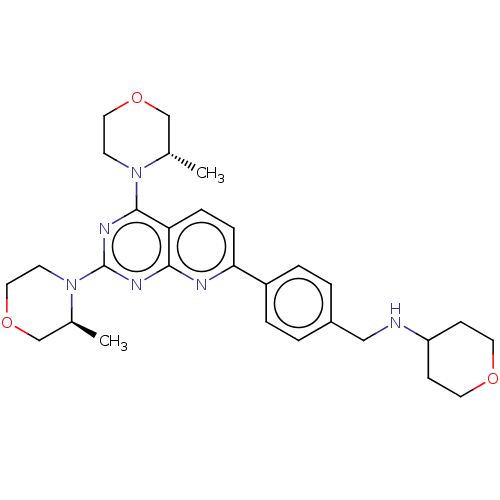 (US9102670, 4e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174472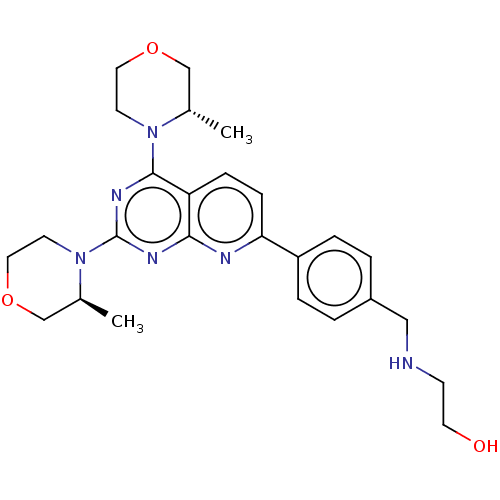 (US9102670, 4f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description For mTOR enzyme activity assays, mTOR protein was isolated from HeLa cell cytoplasmic extract by immunoprecipitation, and activity determined essenti... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50382483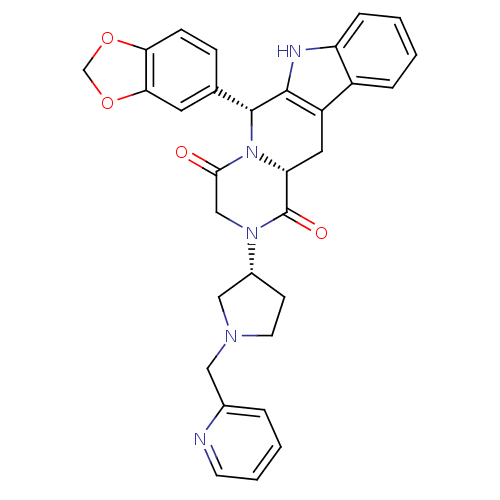 (CHEMBL2022212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of bovine PDE5 after 24 hrs by time-resolved fluorescence resonance energy transfer assay | J Med Chem 55: 1274-86 (2012) Article DOI: 10.1021/jm201422e BindingDB Entry DOI: 10.7270/Q2WW7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174293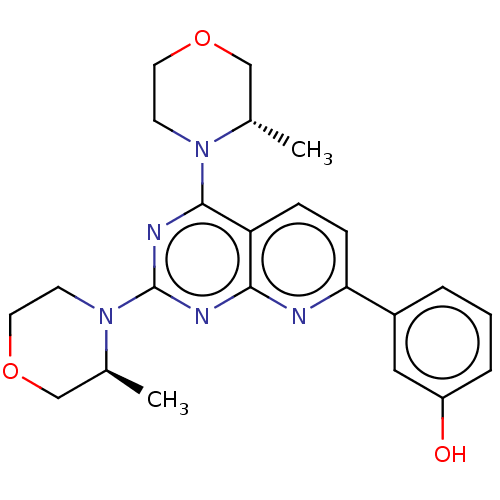 (US9102670, 1h) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174289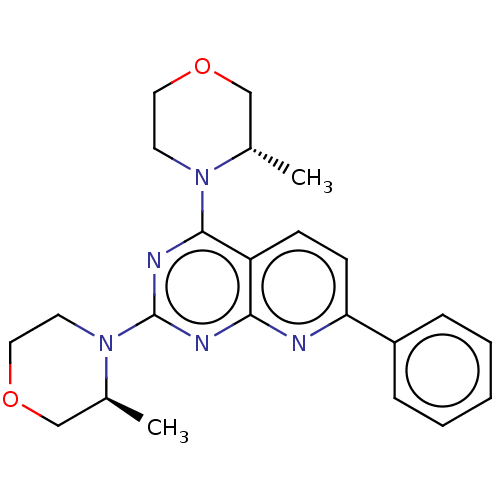 (US9102670, 1d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description For mTOR enzyme activity assays, mTOR protein was isolated from HeLa cell cytoplasmic extract by immunoprecipitation, and activity determined essenti... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174686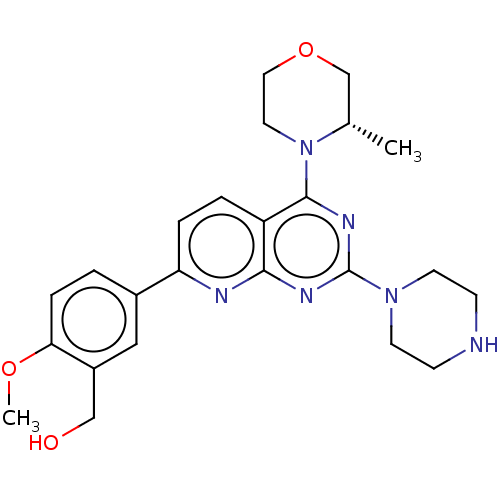 (US9102670, 18ca) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174413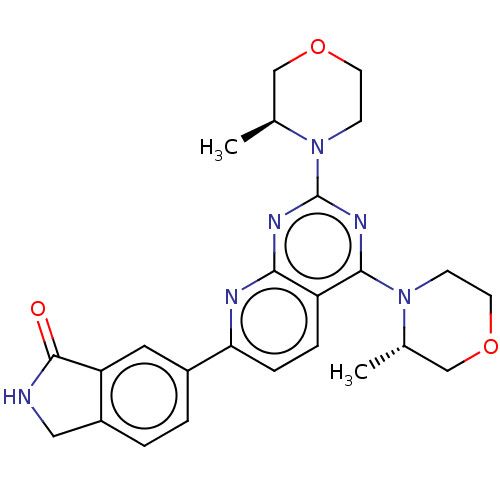 (US9102670, 1dt) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174347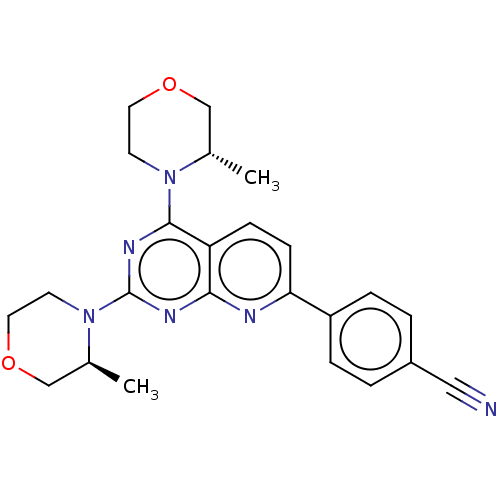 (US9102670, 1bl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174472 (US9102670, 4f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <2.70 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50382482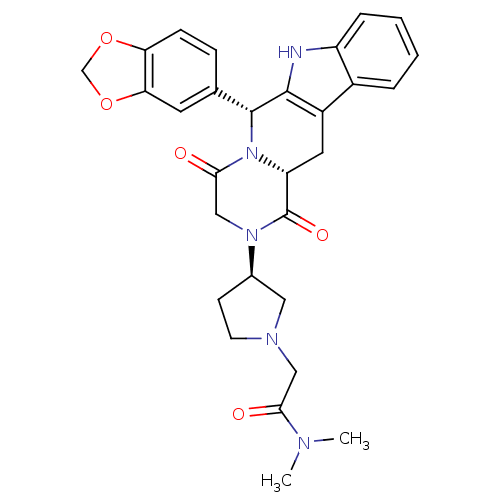 (CHEMBL2022214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of bovine PDE5 after 24 hrs by time-resolved fluorescence resonance energy transfer assay | J Med Chem 55: 1274-86 (2012) Article DOI: 10.1021/jm201422e BindingDB Entry DOI: 10.7270/Q2WW7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174712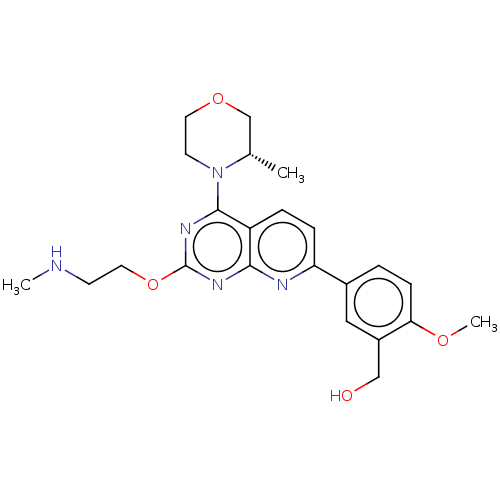 (US9102670, 18db) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50429706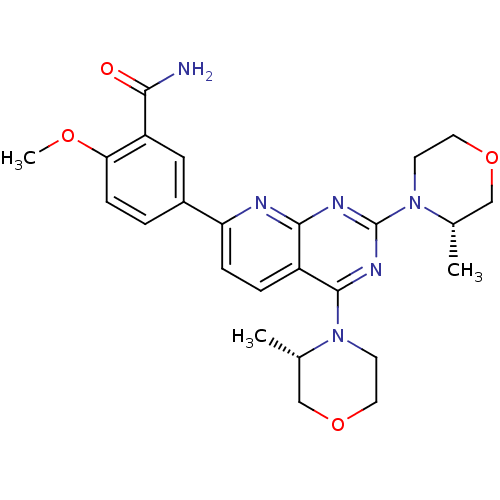 (CHEMBL2336320 | US9102670, 14b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174418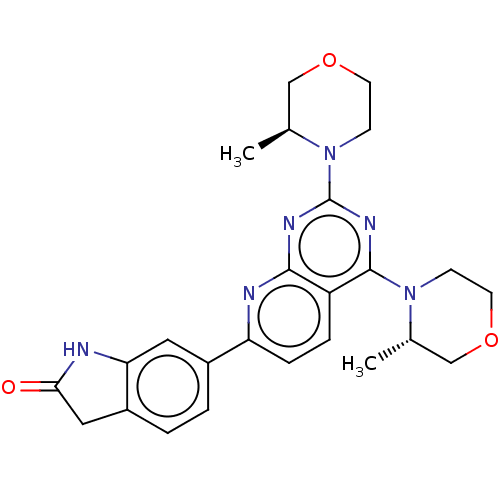 (US9102670, 1dy) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174514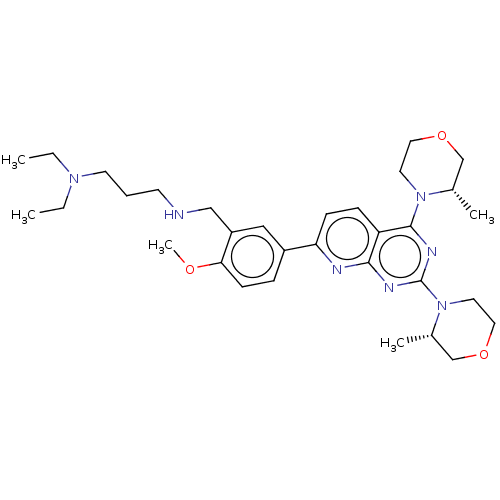 (US9102670, 4av) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-degrading enzyme (Homo sapiens (Human)) | BDBM50525490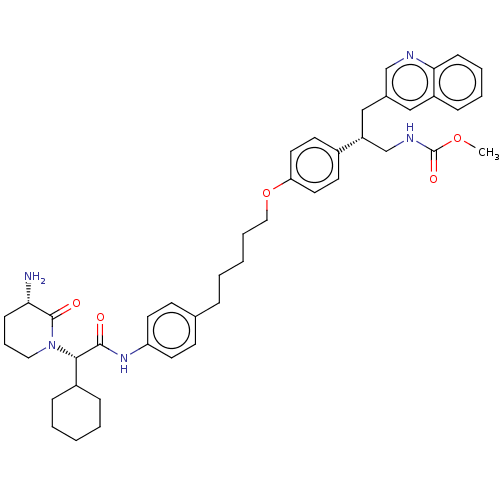 (CHEMBL4437643) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille Curated by ChEMBL | Assay Description Inhibition of recombinant IDE exosite (unknown origin) expressed in Escherichia coli using insulin as substrate incubated for 4 hrs by AlphaLisa assa... | Eur J Med Chem 179: 557-566 (2019) Article DOI: 10.1016/j.ejmech.2019.06.057 BindingDB Entry DOI: 10.7270/Q2XS5ZT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174602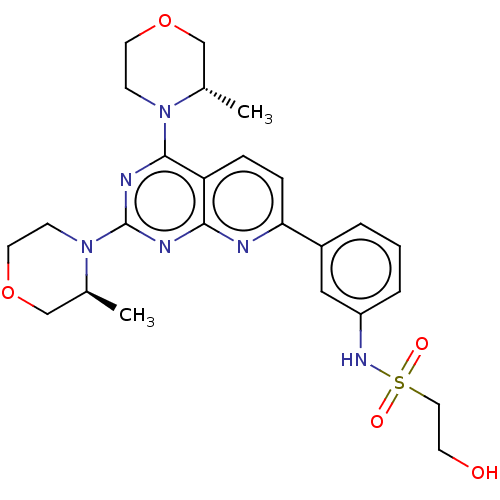 (US9102670, 15a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174290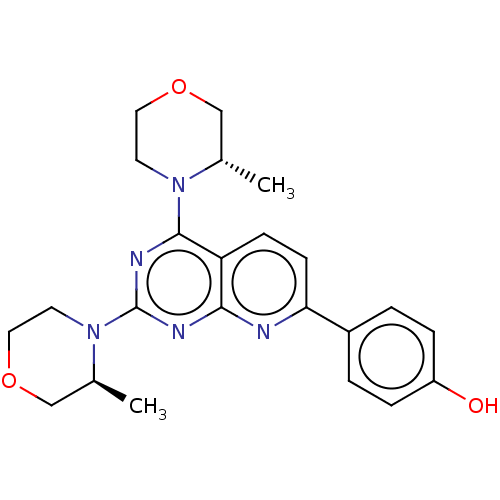 (US9102670, 1e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174360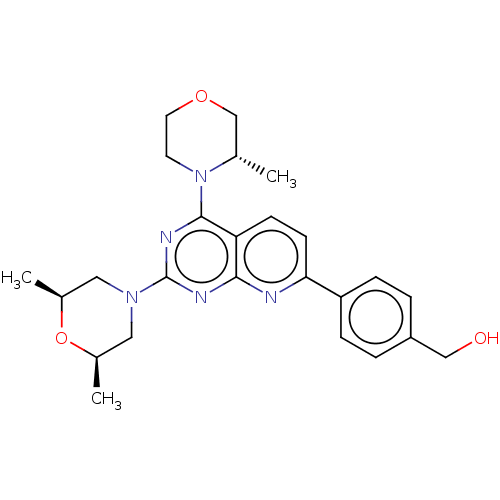 (US9102670, 1by) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174703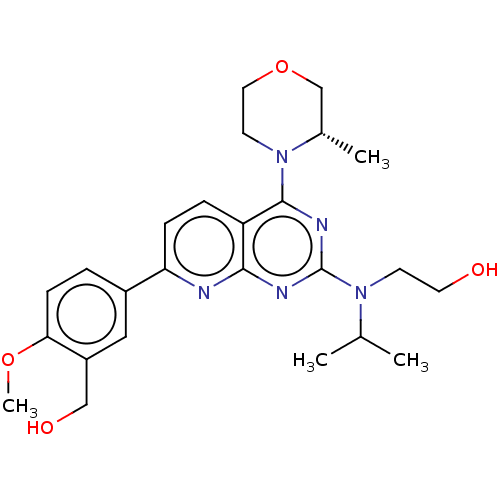 (US9102670, 18cs) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174439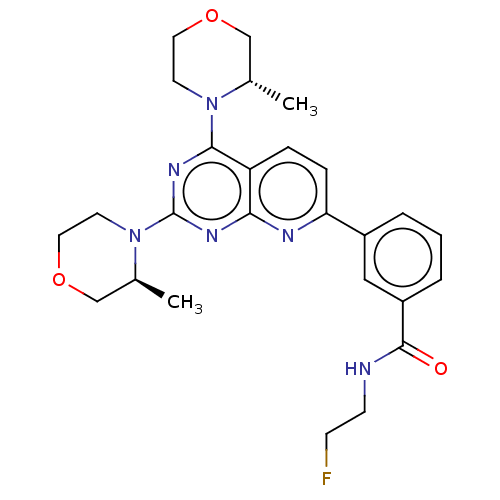 (US9102670, 3e) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174424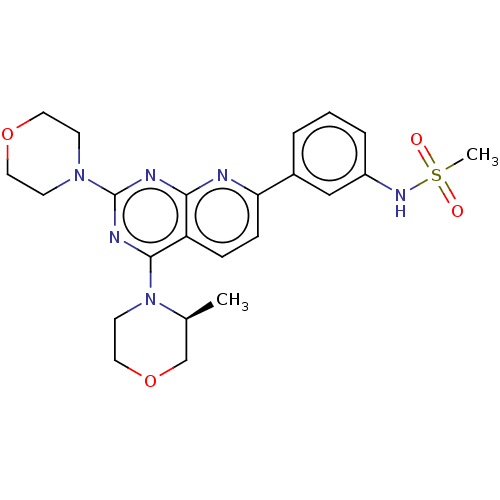 (US9102670, 1ee) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174767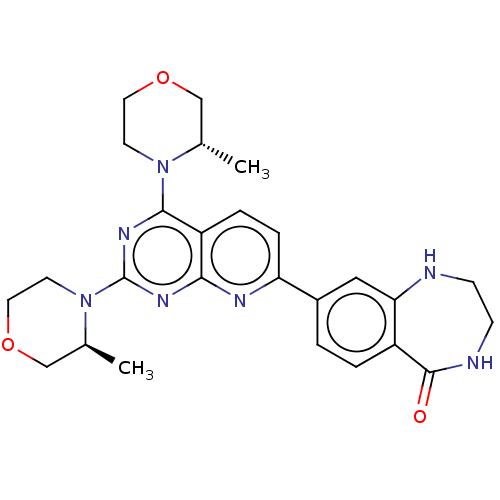 (US9102670, 1de) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174291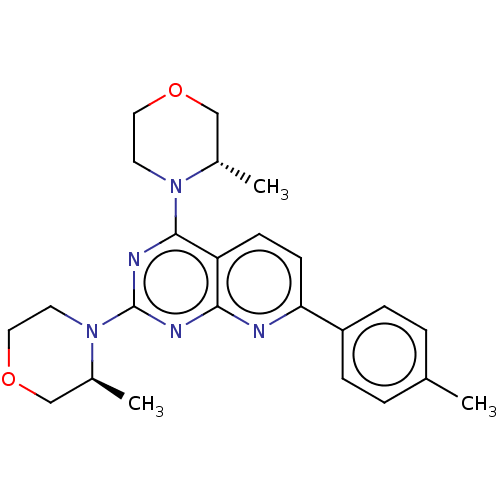 (US9102670, 1f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM14777 ((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of bovine PDE5 after 24 hrs by time-resolved fluorescence resonance energy transfer assay | J Med Chem 55: 1274-86 (2012) Article DOI: 10.1021/jm201422e BindingDB Entry DOI: 10.7270/Q2WW7JPW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174357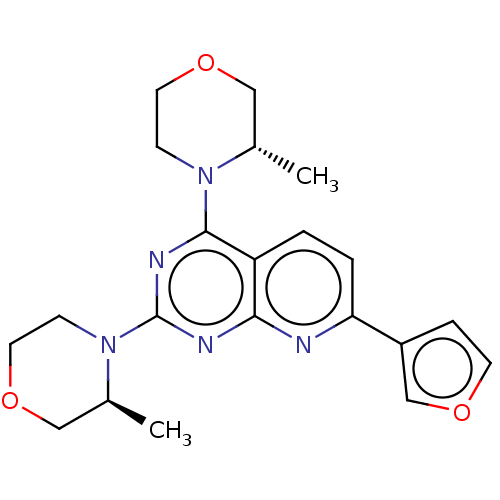 (US9102670, 1bv) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50126458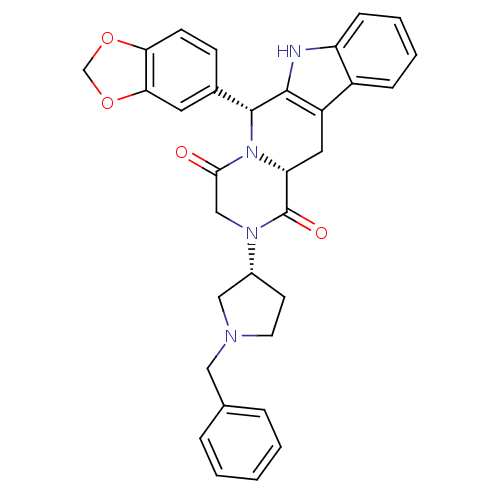 ((6R,12aR)-6-Benzo[1,3]dioxol-5-yl-2-((R)-1-benzyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of bovine PDE5 after 24 hrs by time-resolved fluorescence resonance energy transfer assay | J Med Chem 55: 1274-86 (2012) Article DOI: 10.1021/jm201422e BindingDB Entry DOI: 10.7270/Q2WW7JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174699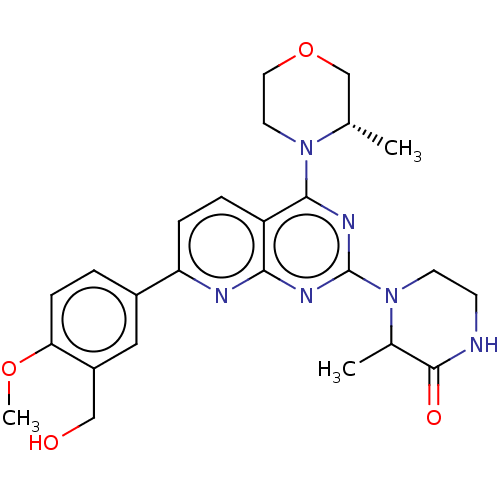 (US9102670, 18co) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174441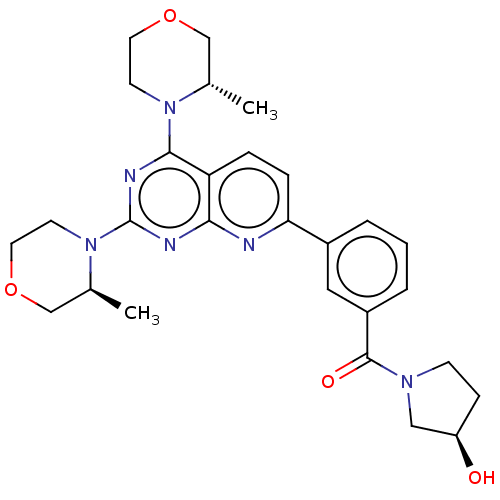 (US9102670, 3g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM60569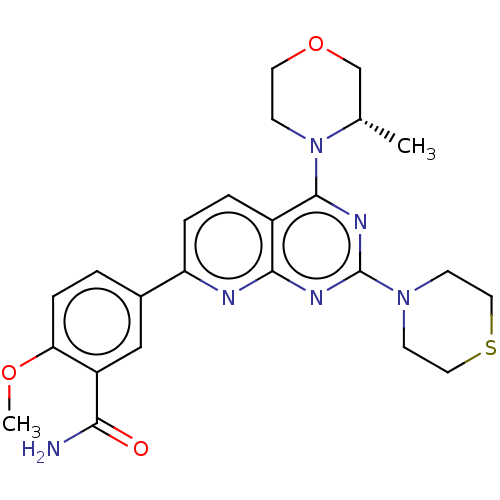 (US9102670, 1dn) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174294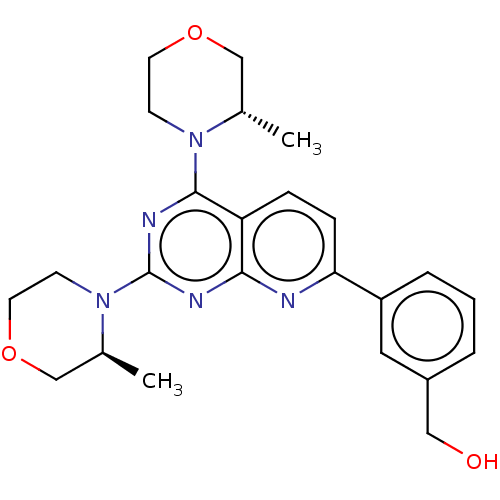 (US9102670, 1i) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174700 (US9102670, 18cp) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174296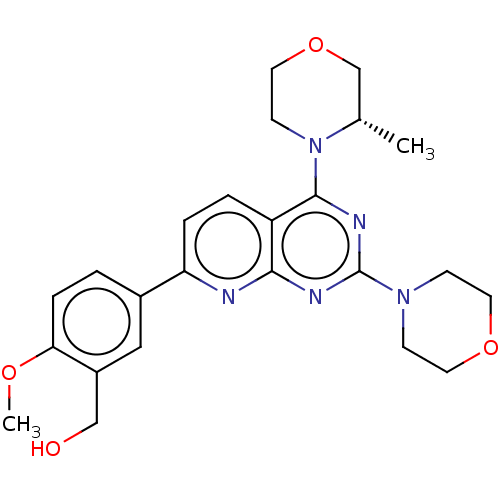 (US9102670, 1l) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174327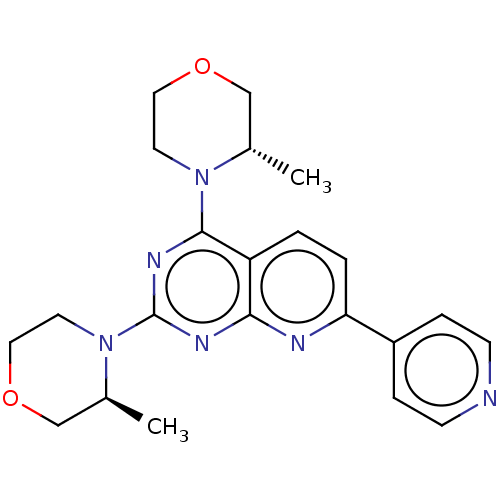 (US9102670, 1ar) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174702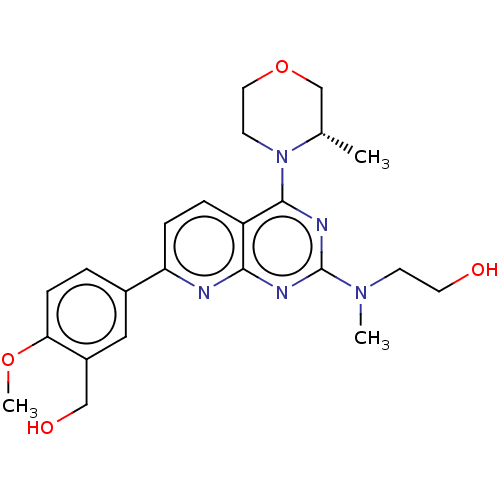 (US9102670, 18cr) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174364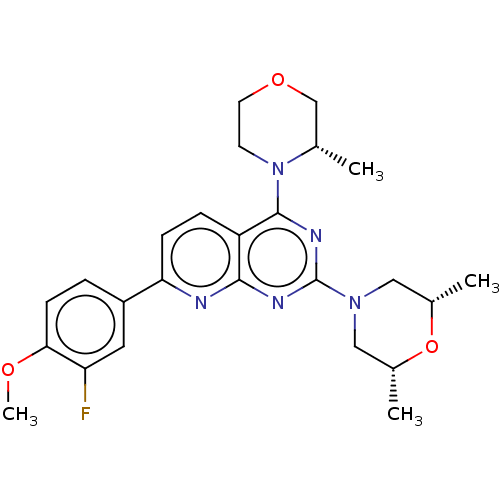 (US9102670, 1cc) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM60571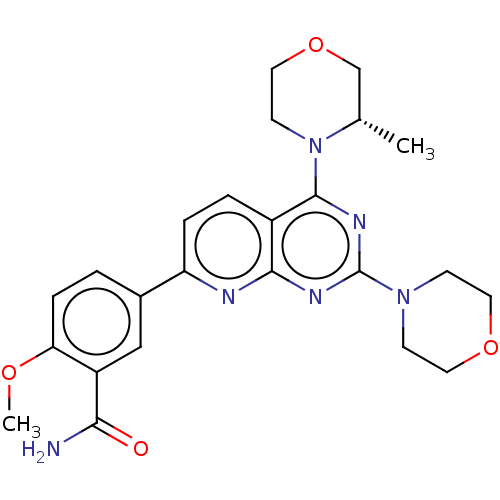 (US9102670, 1dp) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM60572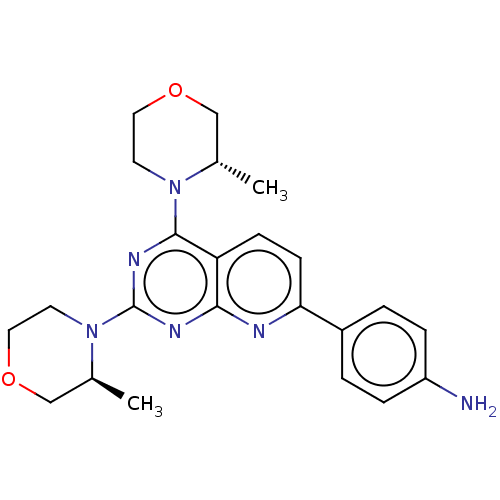 (US9102670, 1az) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.87 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM174314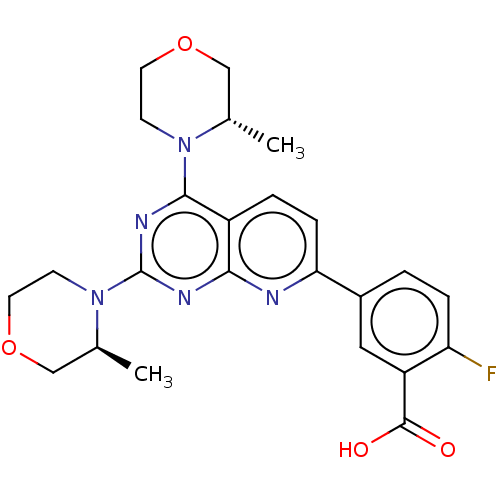 (US9102670, 1ad) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | 25 |
KUDOS PHARMACEUTICALS LIMITED US Patent | Assay Description The assay used AlphaScreen technology (Gray et al., Analytical Biochemistry, 2003, 313: 234-245) to determine the ability of test compounds to inhibi... | US Patent US9102670 (2015) BindingDB Entry DOI: 10.7270/Q26M35MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM109086 (US10793535, Cmpd ID 727 | US8604016, 670 | US99382...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM50514979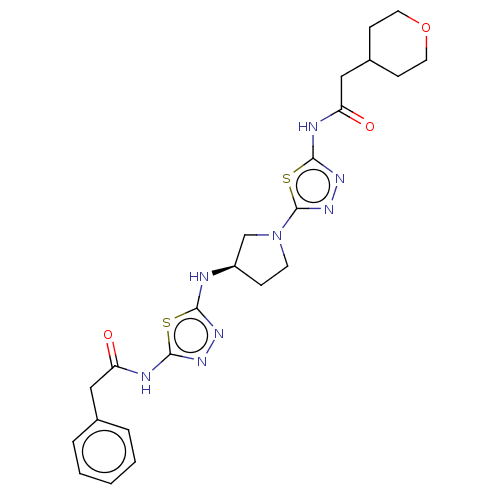 (CHEMBL4457936) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... | J Med Chem 62: 6540-6560 (2019) Article DOI: 10.1021/acs.jmedchem.9b00260 BindingDB Entry DOI: 10.7270/Q2QN6B4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1162 total ) | Next | Last >> |