

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based s... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based ... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50190650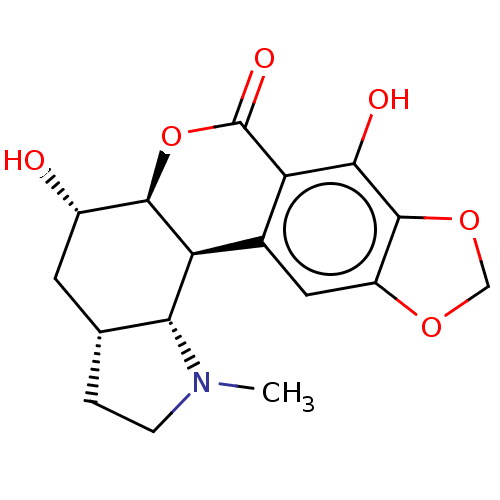 (CHEMBL3906484) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based ... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50190650 (CHEMBL3906484) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based s... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50221066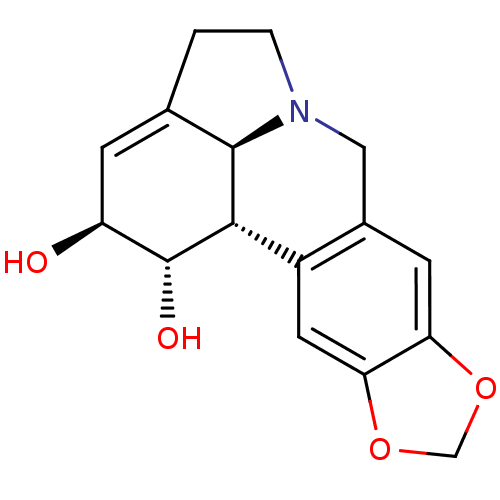 ((-)-lycorine | 9,10-(methylenedioxy)-3,12-didehydr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based s... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50190649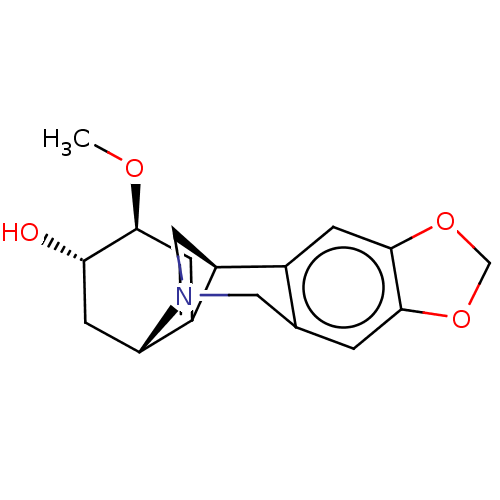 (Montanine) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based s... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50190651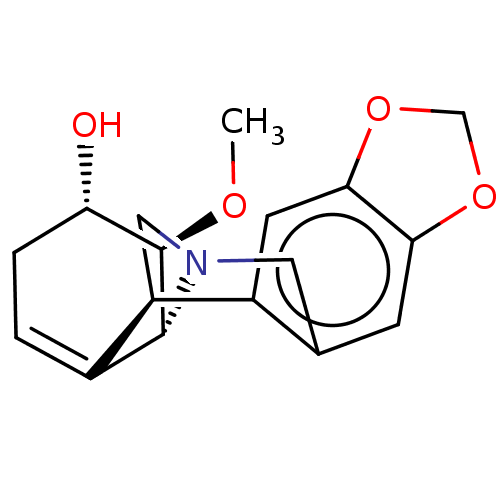 (CHEMBL3956367) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based s... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50190649 (Montanine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based ... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50190651 (CHEMBL3956367) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based ... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50190648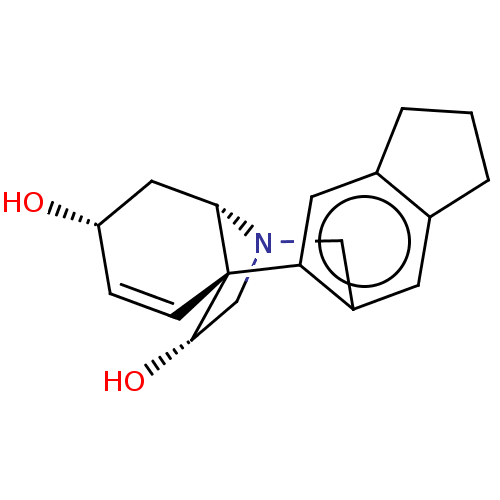 (CHEMBL3889595) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based ... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50190647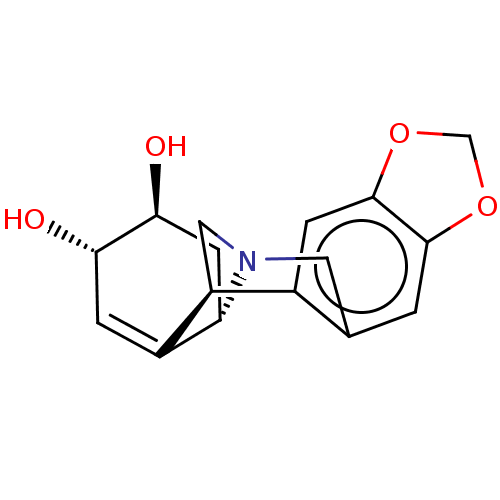 (CHEMBL3978970) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based ... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50190648 (CHEMBL3889595) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based s... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50190647 (CHEMBL3978970) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based s... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50221066 ((-)-lycorine | 9,10-(methylenedioxy)-3,12-didehydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de San Juan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using ATCI as substrate preincubated for 30 mins followed by substrate addition measured after 5 mins by DTNB-based ... | J Nat Prod 79: 1241-8 (2016) Article DOI: 10.1021/acs.jnatprod.5b00785 BindingDB Entry DOI: 10.7270/Q2WM1GBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50510090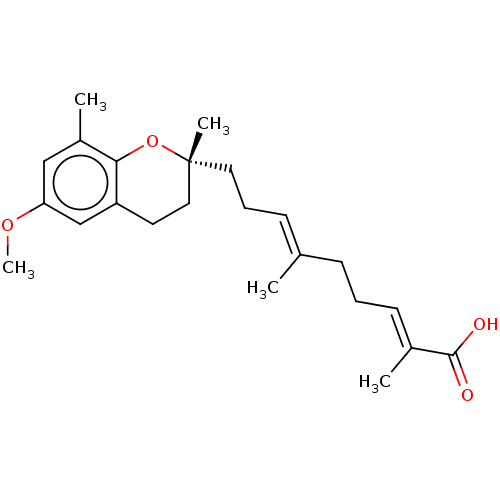 (CHEMBL4535730) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
University of Valencia Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha expressed in African green monkey COS7 cells assessed as increase in receptor transcriptional activity by lucifer... | J Nat Prod 82: 1802-1812 (2019) Article DOI: 10.1021/acs.jnatprod.9b00003 BindingDB Entry DOI: 10.7270/Q2ZW1Q7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM24566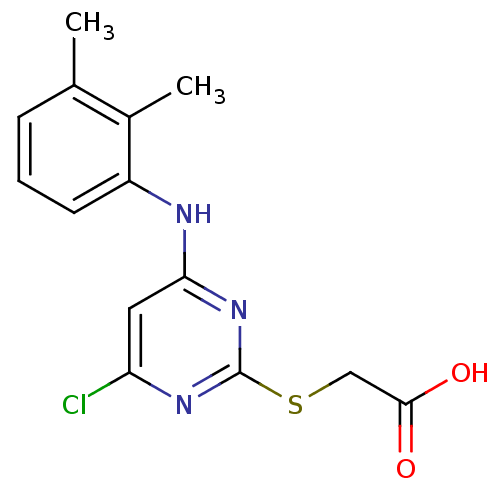 (2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
University of Valencia Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha expressed in African green monkey COS7 cells assessed as increase in receptor transcriptional activity by lucifer... | J Nat Prod 82: 1802-1812 (2019) Article DOI: 10.1021/acs.jnatprod.9b00003 BindingDB Entry DOI: 10.7270/Q2ZW1Q7G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50510090 (CHEMBL4535730) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
University of Valencia Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma expressed in African green monkey COS7 cells assessed as increase in receptor transcriptional activity by lucifer... | J Nat Prod 82: 1802-1812 (2019) Article DOI: 10.1021/acs.jnatprod.9b00003 BindingDB Entry DOI: 10.7270/Q2ZW1Q7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50510091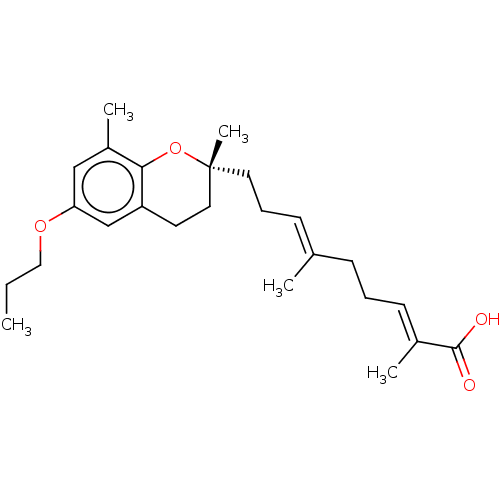 (CHEMBL4519542) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a |
University of Valencia Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma expressed in African green monkey COS7 cells assessed as increase in receptor transcriptional activity by lucifer... | J Nat Prod 82: 1802-1812 (2019) Article DOI: 10.1021/acs.jnatprod.9b00003 BindingDB Entry DOI: 10.7270/Q2ZW1Q7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50510092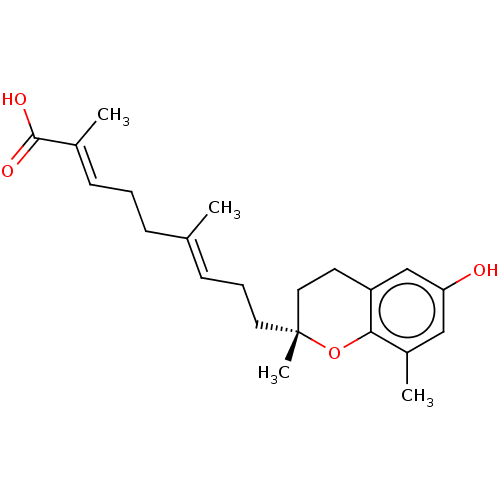 (CHEMBL4593987) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
University of Valencia Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma expressed in African green monkey COS7 cells assessed as increase in receptor transcriptional activity by lucifer... | J Nat Prod 82: 1802-1812 (2019) Article DOI: 10.1021/acs.jnatprod.9b00003 BindingDB Entry DOI: 10.7270/Q2ZW1Q7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50510092 (CHEMBL4593987) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 184 | n/a | n/a | n/a | n/a |
University of Valencia Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha expressed in African green monkey COS7 cells assessed as increase in receptor transcriptional activity by lucifer... | J Nat Prod 82: 1802-1812 (2019) Article DOI: 10.1021/acs.jnatprod.9b00003 BindingDB Entry DOI: 10.7270/Q2ZW1Q7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50510091 (CHEMBL4519542) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
University of Valencia Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha expressed in African green monkey COS7 cells assessed as increase in receptor transcriptional activity by lucifer... | J Nat Prod 82: 1802-1812 (2019) Article DOI: 10.1021/acs.jnatprod.9b00003 BindingDB Entry DOI: 10.7270/Q2ZW1Q7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50510093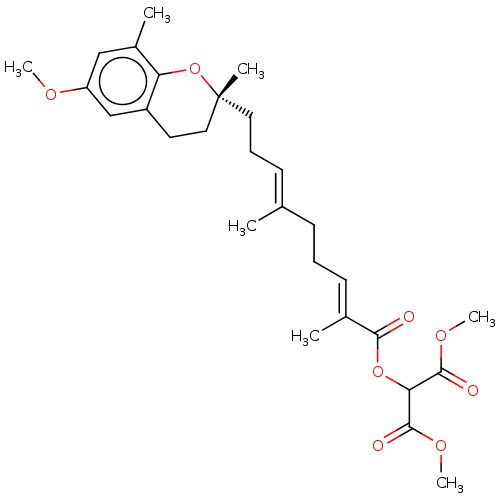 (CHEMBL4466552) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a |
University of Valencia Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha expressed in African green monkey COS7 cells assessed as increase in receptor transcriptional activity by lucifer... | J Nat Prod 82: 1802-1812 (2019) Article DOI: 10.1021/acs.jnatprod.9b00003 BindingDB Entry DOI: 10.7270/Q2ZW1Q7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50510094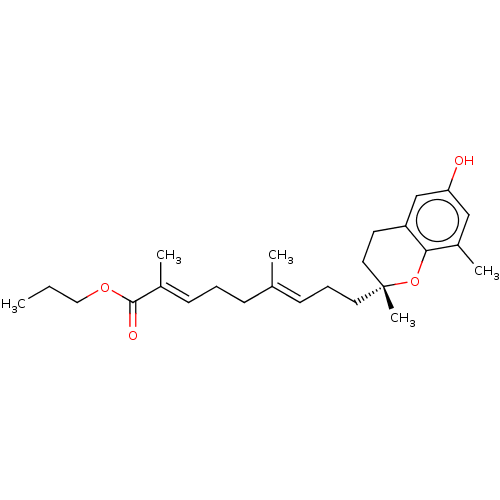 (CHEMBL4460072) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 991 | n/a | n/a | n/a | n/a |
University of Valencia Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma expressed in African green monkey COS7 cells assessed as increase in receptor transcriptional activity by lucifer... | J Nat Prod 82: 1802-1812 (2019) Article DOI: 10.1021/acs.jnatprod.9b00003 BindingDB Entry DOI: 10.7270/Q2ZW1Q7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50030474 (Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
University of Valencia Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma expressed in African green monkey COS7 cells assessed as increase in receptor transcriptional activity by lucifer... | J Nat Prod 82: 1802-1812 (2019) Article DOI: 10.1021/acs.jnatprod.9b00003 BindingDB Entry DOI: 10.7270/Q2ZW1Q7G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||