

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485688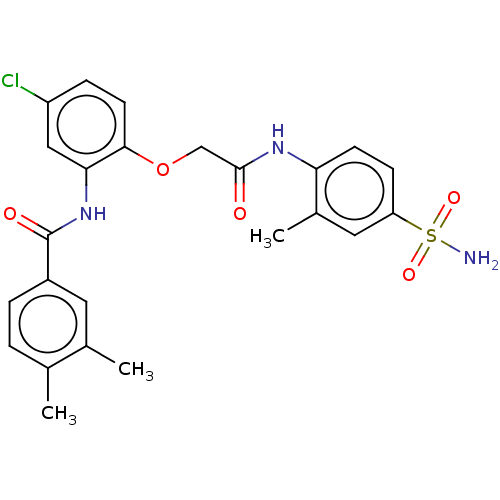 (CHEMBL2151836) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase activity using poly (rA)/oligo (dT)15 homopolymer template after 1 hr by ELISA | Eur J Med Chem 58: 504-12 (2012) Article DOI: 10.1016/j.ejmech.2012.03.032 BindingDB Entry DOI: 10.7270/Q2K07747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183273 ((S)-6-(3-chloro-2-fluorobenzyl)-1-(1-hydroxy-3-met...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase | Bioorg Med Chem 23: 3860-8 (2015) Article DOI: 10.1016/j.bmc.2015.03.037 BindingDB Entry DOI: 10.7270/Q2FX7DFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50388686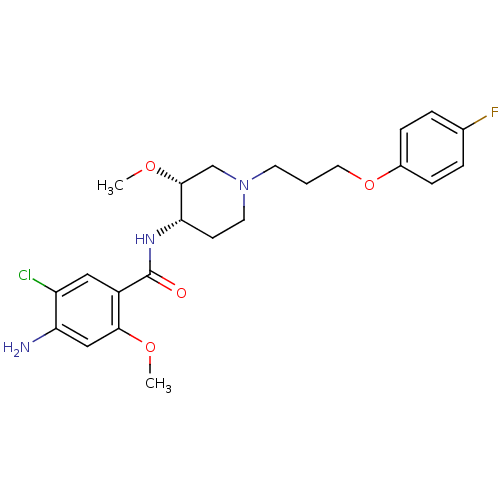 (CHEMBL74656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00468 BindingDB Entry DOI: 10.7270/Q29027TT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50485689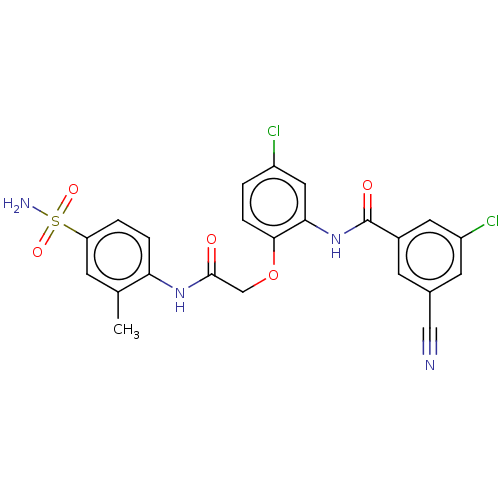 (CHEMBL2151844) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase activity using poly (rA)/oligo (dT)15 homopolymer template after 1 hr by ELISA | Eur J Med Chem 58: 504-12 (2012) Article DOI: 10.1016/j.ejmech.2012.03.032 BindingDB Entry DOI: 10.7270/Q2K07747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478026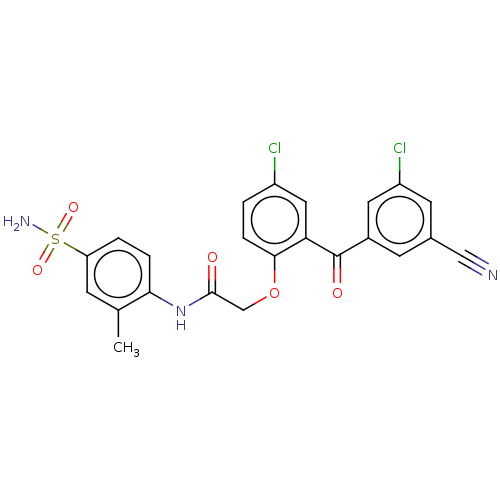 (CHEMBL203420 | GW678248 | GW8248) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 reverse transcriptase activity using poly (rA)/oligo (dT)15 homopolymer template after 1 hr by ELISA | Eur J Med Chem 58: 504-12 (2012) Article DOI: 10.1016/j.ejmech.2012.03.032 BindingDB Entry DOI: 10.7270/Q2K07747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50420191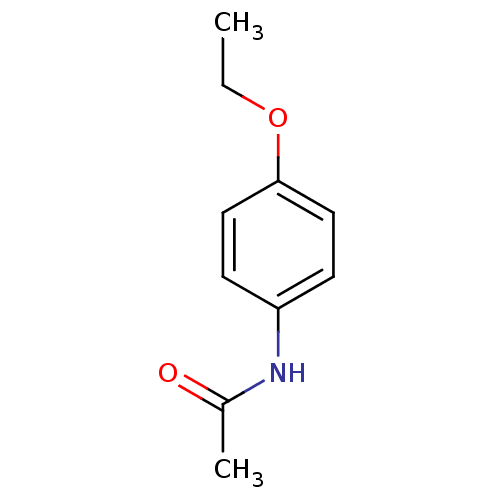 (ACETPHENETIDIN | Acetophenetidin | PHENACETIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114512 BindingDB Entry DOI: 10.7270/Q2TH8RR7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50504890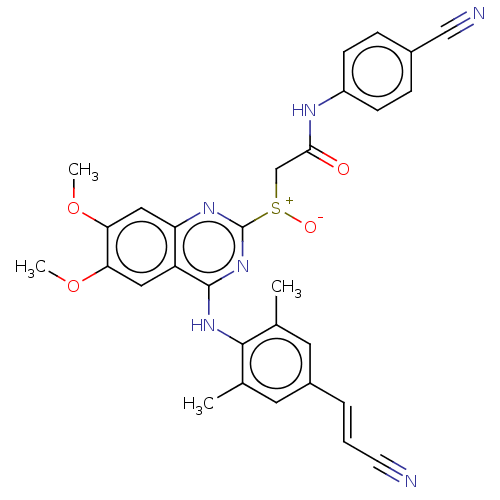 (CHEMBL4463378) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV-1 His-tagged reverse transcriptase p66/p51 expressed in Escherichia coli JM109 using poly(rA)/oligo(dT)16 as ... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111874 BindingDB Entry DOI: 10.7270/Q20P1395 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50580775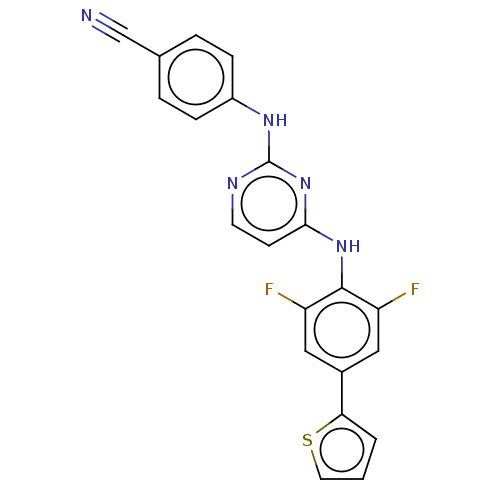 (CHEMBL5094057) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50580779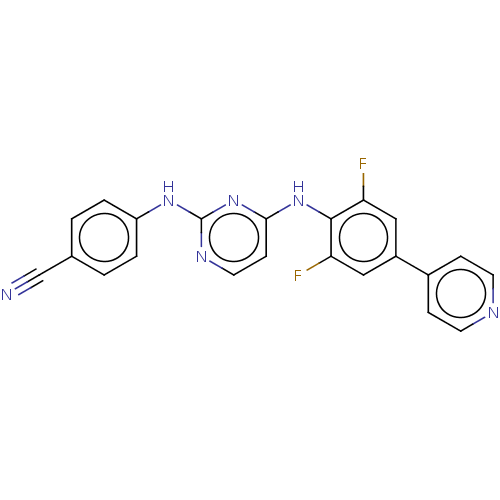 (CHEMBL5076799) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50580789 (CHEMBL5075564) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00468 BindingDB Entry DOI: 10.7270/Q29027TT | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584634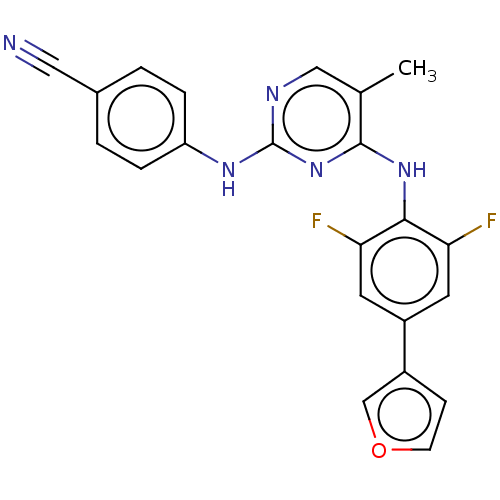 (CHEMBL5078684) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50580784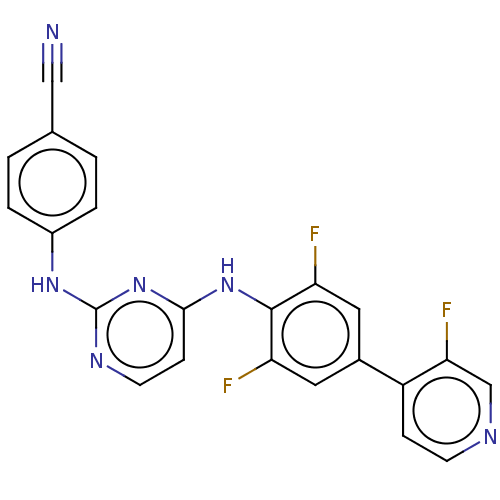 (CHEMBL5086481) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50580774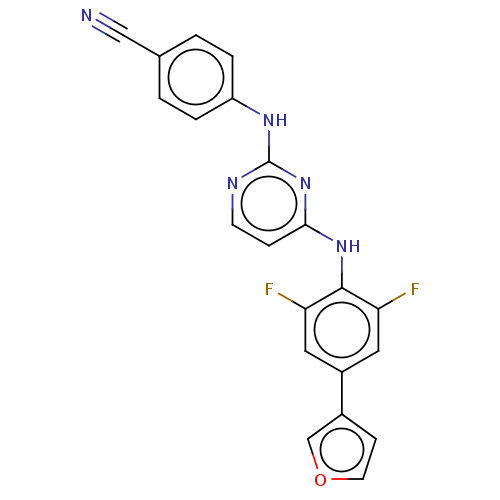 (CHEMBL5090703) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50580773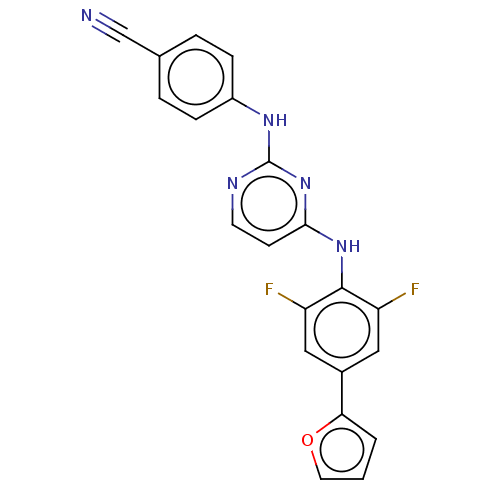 (CHEMBL5083504) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00128 BindingDB Entry DOI: 10.7270/Q2Q81HT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase F227L/V106A double mutant using oligo(dT)16 as primer measured after 40 mins by picogreen-dye based spectrof... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113868 BindingDB Entry DOI: 10.7270/Q2NS0ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase E138K mutant using oligo(dT)16 as primer measured after 40 mins by picogreen-dye based spectrofluorimetric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113868 BindingDB Entry DOI: 10.7270/Q2NS0ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase Y181C mutant using oligo(dT)16 as primer measured after 40 mins by picogreen-dye based spectrofluorimetric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113868 BindingDB Entry DOI: 10.7270/Q2NS0ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase L100I mutant using oligo(dT)16 as primer measured after 40 mins by picogreen-dye based spectrofluorimetric a... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113868 BindingDB Entry DOI: 10.7270/Q2NS0ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant using oligo(dT)16 as primer measured after 40 mins by picogreen-dye based spectrof... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113868 BindingDB Entry DOI: 10.7270/Q2NS0ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 reverse transcriptase using oligo(dT)16 as primer measured after 40 mins by picogreen-dye based spectrofluorimetric anal... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113868 BindingDB Entry DOI: 10.7270/Q2NS0ZSB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114512 BindingDB Entry DOI: 10.7270/Q2TH8RR7 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00468 BindingDB Entry DOI: 10.7270/Q29027TT | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 Reverse transcriptase p66/p51 assessed as relative fluorescence signal after 40 mins | Bioorg Med Chem 23: 3860-8 (2015) Article DOI: 10.1016/j.bmc.2015.03.037 BindingDB Entry DOI: 10.7270/Q2FX7DFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50504892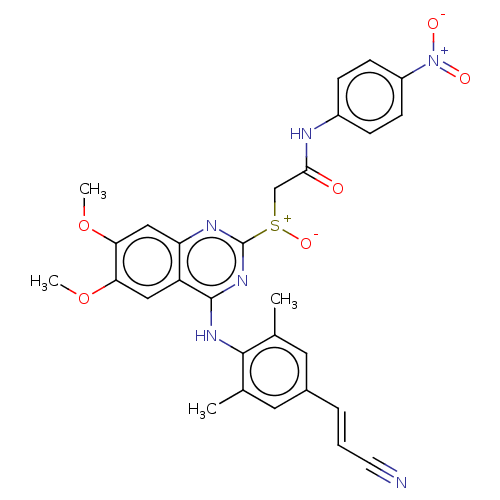 (CHEMBL4440030) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV-1 His-tagged reverse transcriptase p66/p51 expressed in Escherichia coli JM109 using poly(rA)/oligo(dT)16 as ... | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111874 BindingDB Entry DOI: 10.7270/Q20P1395 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00128 BindingDB Entry DOI: 10.7270/Q2Q81HT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50580776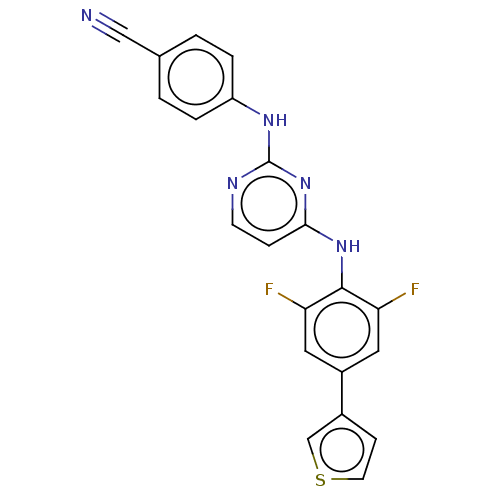 (CHEMBL5083415) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50580783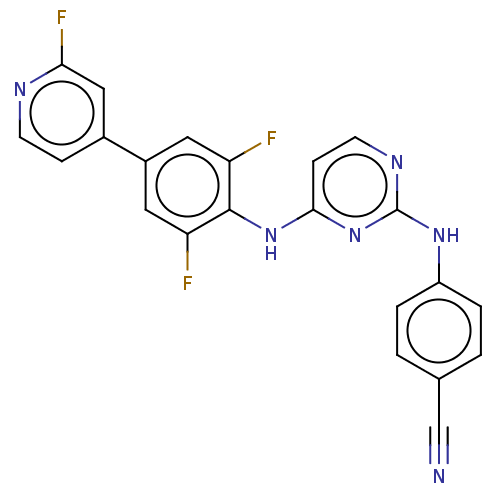 (CHEMBL5086710) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50580790 (CHEMBL5086832) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584635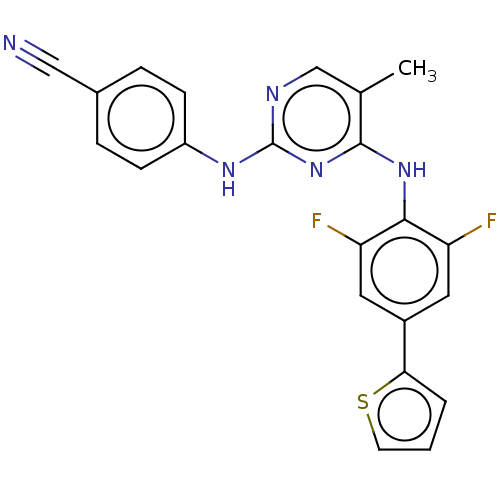 (CHEMBL5094298) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584633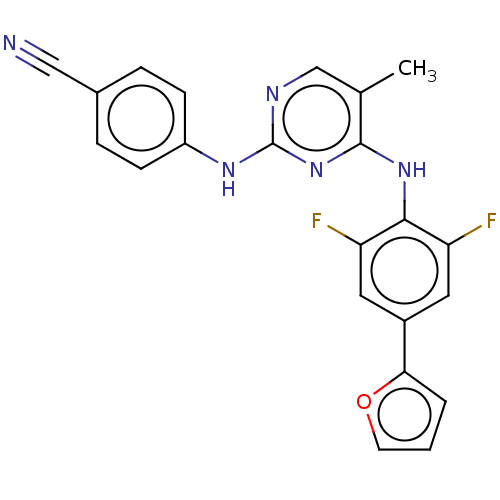 (CHEMBL5076567) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584630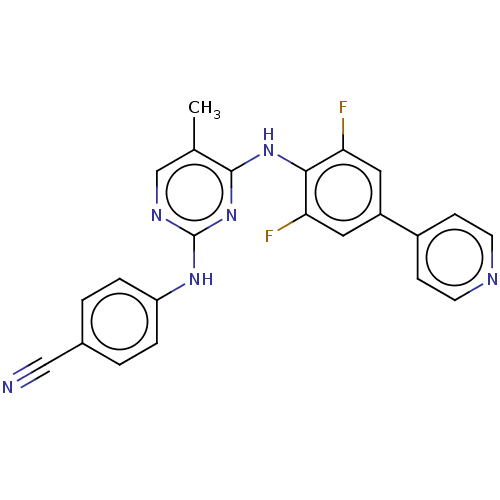 (CHEMBL5086160) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584636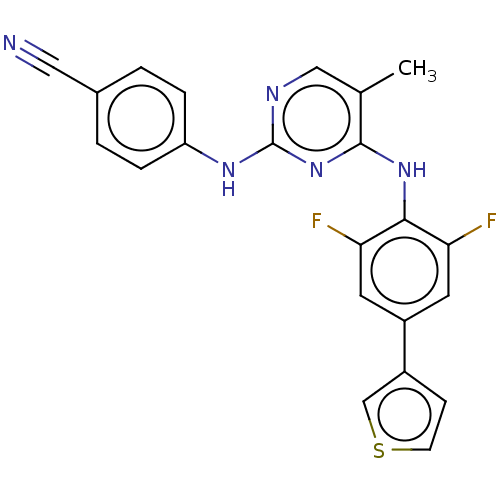 (CHEMBL5077589) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00468 BindingDB Entry DOI: 10.7270/Q29027TT | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50580787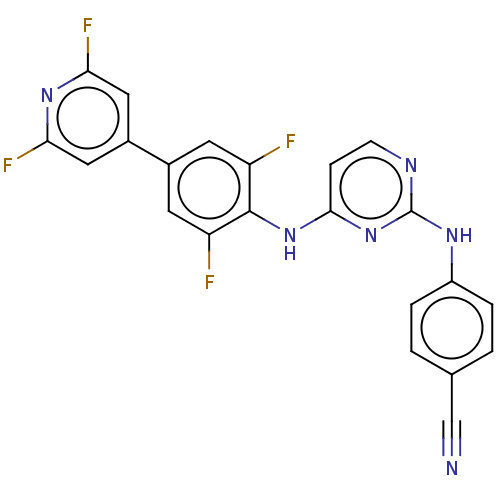 (CHEMBL5075410) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50566509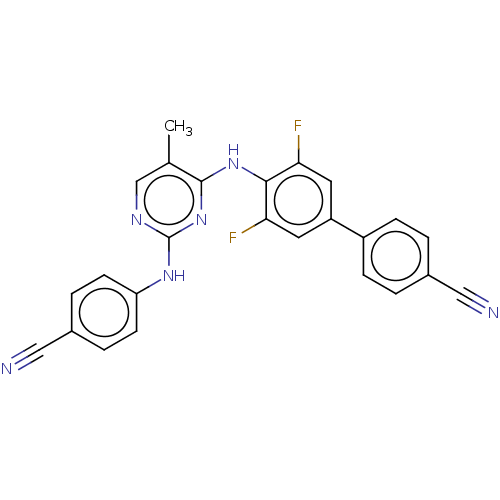 (CHEMBL4845938) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00128 BindingDB Entry DOI: 10.7270/Q2Q81HT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50580782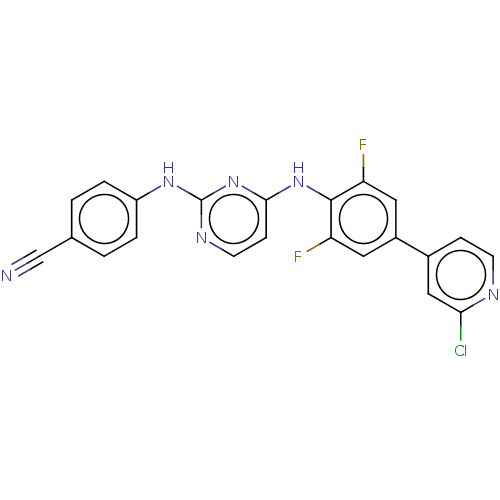 (CHEMBL5090452) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM222178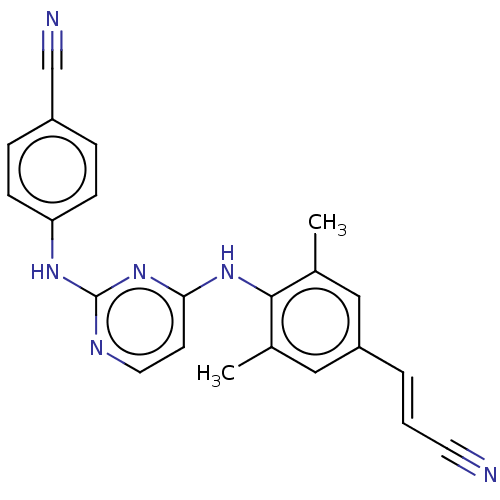 (Rilpivirine) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50566501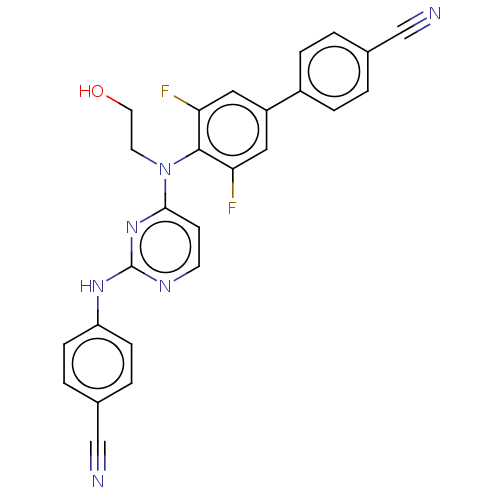 (CHEMBL4868044) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00128 BindingDB Entry DOI: 10.7270/Q2Q81HT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50566498 (CHEMBL4848764) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00128 BindingDB Entry DOI: 10.7270/Q2Q81HT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50580788 (CHEMBL5087811) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild-type HIV1 p66/p51 reverse transcriptase assessed as inhibition of [3H]dGTP incorporation using poly (rA) as templates ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00708 BindingDB Entry DOI: 10.7270/Q2S186CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 reverse transcriptase p66/p51 after 40 mins by spectrophotometry | Bioorg Med Chem 22: 2535-41 (2014) Article DOI: 10.1016/j.bmc.2014.02.030 BindingDB Entry DOI: 10.7270/Q2K93BH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type HIV-1 reverse transcriptase p66/p51 expressed in Escherichia coli JM109 using poly(rA)/oligo(dT)16 (1:1.2) as tem... | Bioorg Med Chem 21: 6477-83 (2013) Article DOI: 10.1016/j.bmc.2013.08.040 BindingDB Entry DOI: 10.7270/Q2KK9FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50582100 (CHEMBL5073811) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00468 BindingDB Entry DOI: 10.7270/Q29027TT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50582100 (CHEMBL5073811) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type HIV1 reverse transcriptase using oligo(dT)16 as primer measured after 40 mins by picogreen-dye based spectrofluorimetric anal... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113868 BindingDB Entry DOI: 10.7270/Q2NS0ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 638 total ) | Next | Last >> |