 Found 109 hits with Last Name = 'piedrafita' and Initial = 'fj'
Found 109 hits with Last Name = 'piedrafita' and Initial = 'fj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Catechol O-methyltransferase
(Sus scrofa) | BDBM50004044

(3-Hydroxy-4-methoxy-5-nitro-benzaldehyde | CHEMBL8...)Show InChI InChI=1S/C8H7NO5/c1-14-8-6(9(12)13)2-5(4-10)3-7(8)11/h2-4,11H,1H3 | MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Orgánica General (CSIC)
Curated by ChEMBL
| Assay Description
Compound was evaluated for reversible inhibition of Catechol O-methyltransferase with variable AdoMet and saturating catechol as substrate (mixed inh... |
J Med Chem 35: 4584-8 (1993)
BindingDB Entry DOI: 10.7270/Q2X63KWW |
More data for this
Ligand-Target Pair | |
Catechol O-methyltransferase
(Sus scrofa) | BDBM50004044

(3-Hydroxy-4-methoxy-5-nitro-benzaldehyde | CHEMBL8...)Show InChI InChI=1S/C8H7NO5/c1-14-8-6(9(12)13)2-5(4-10)3-7(8)11/h2-4,11H,1H3 | MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Orgánica General (CSIC)
Curated by ChEMBL
| Assay Description
Compound was evaluated for reversible inhibition of Catechol O-methyltransferase with saturating AdoMet and variable catechol as substrate (noncompet... |
J Med Chem 35: 4584-8 (1993)
BindingDB Entry DOI: 10.7270/Q2X63KWW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase LMTK3
(Homo sapiens) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
San Diego State University
Curated by ChEMBL
| Assay Description
Inhibition of LMTK3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127108
BindingDB Entry DOI: 10.7270/Q26M3BDB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50614535

(CHEMBL5271284)Show SMILES Cc1cc(Oc2ccccc2)ccc1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50614535

(CHEMBL5271284)Show SMILES Cc1cc(Oc2ccccc2)ccc1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50614535

(CHEMBL5271284)Show SMILES Cc1cc(Oc2ccccc2)ccc1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50614536
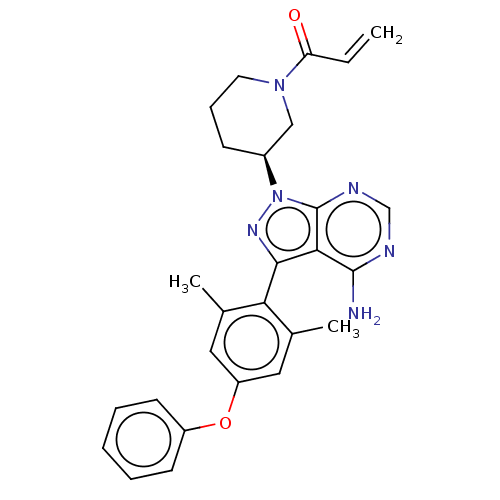
(CHEMBL5278149)Show SMILES Cc1cc(Oc2ccccc2)cc(C)c1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r,wD:18.19,(1.35,.46,;.58,1.79,;1.37,3.12,;.61,4.46,;1.39,5.78,;2.93,5.76,;3.72,7.09,;5.27,7.08,;6.02,5.73,;5.23,4.4,;3.69,4.43,;-.93,4.47,;-1.71,3.14,;-3.25,3.14,;-.96,1.81,;-1.65,.43,;-.93,-.93,;-2.01,-2.04,;-1.75,-3.56,;-2.85,-4.63,;-2.48,-6.12,;-1,-6.55,;.11,-5.48,;-.26,-3.98,;1.59,-5.89,;1.9,-7.09,;2.7,-4.83,;3.88,-5.17,;-3.38,-1.35,;-4.81,-1.92,;-6.02,-.97,;-5.8,.55,;-4.37,1.12,;-4.2,2.34,;-3.16,.17,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50614536

(CHEMBL5278149)Show SMILES Cc1cc(Oc2ccccc2)cc(C)c1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r,wD:18.19,(1.35,.46,;.58,1.79,;1.37,3.12,;.61,4.46,;1.39,5.78,;2.93,5.76,;3.72,7.09,;5.27,7.08,;6.02,5.73,;5.23,4.4,;3.69,4.43,;-.93,4.47,;-1.71,3.14,;-3.25,3.14,;-.96,1.81,;-1.65,.43,;-.93,-.93,;-2.01,-2.04,;-1.75,-3.56,;-2.85,-4.63,;-2.48,-6.12,;-1,-6.55,;.11,-5.48,;-.26,-3.98,;1.59,-5.89,;1.9,-7.09,;2.7,-4.83,;3.88,-5.17,;-3.38,-1.35,;-4.81,-1.92,;-6.02,-.97,;-5.8,.55,;-4.37,1.12,;-4.2,2.34,;-3.16,.17,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50614535

(CHEMBL5271284)Show SMILES Cc1cc(Oc2ccccc2)ccc1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50614535

(CHEMBL5271284)Show SMILES Cc1cc(Oc2ccccc2)ccc1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50614536

(CHEMBL5278149)Show SMILES Cc1cc(Oc2ccccc2)cc(C)c1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r,wD:18.19,(1.35,.46,;.58,1.79,;1.37,3.12,;.61,4.46,;1.39,5.78,;2.93,5.76,;3.72,7.09,;5.27,7.08,;6.02,5.73,;5.23,4.4,;3.69,4.43,;-.93,4.47,;-1.71,3.14,;-3.25,3.14,;-.96,1.81,;-1.65,.43,;-.93,-.93,;-2.01,-2.04,;-1.75,-3.56,;-2.85,-4.63,;-2.48,-6.12,;-1,-6.55,;.11,-5.48,;-.26,-3.98,;1.59,-5.89,;1.9,-7.09,;2.7,-4.83,;3.88,-5.17,;-3.38,-1.35,;-4.81,-1.92,;-6.02,-.97,;-5.8,.55,;-4.37,1.12,;-4.2,2.34,;-3.16,.17,)| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50614535

(CHEMBL5271284)Show SMILES Cc1cc(Oc2ccccc2)ccc1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50447840

(BMS-204493 | CHEMBL472172)Show SMILES CC1(C)CC=C(C#Cc2ccccc2)c2cc(\C=C\c3ccc(cc3)C(O)=O)ccc12 |t:4| Show InChI InChI=1S/C29H24O2/c1-29(2)19-18-24(14-10-21-6-4-3-5-7-21)26-20-23(13-17-27(26)29)9-8-22-11-15-25(16-12-22)28(30)31/h3-9,11-13,15-18,20H,19H2,1-2H3,(H,30,31)/b9-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RARalpha (unknown origin) |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50614535

(CHEMBL5271284)Show SMILES Cc1cc(Oc2ccccc2)ccc1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase LMTK3
(Homo sapiens) | BDBM50542351
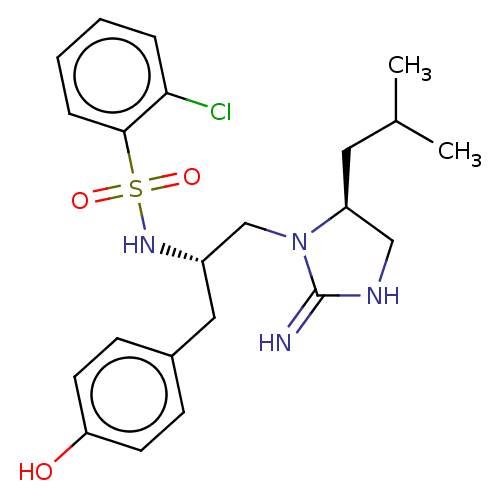
(CHEMBL4637017)Show SMILES CC(C)C[C@H]1CNC(=N)N1C[C@H](Cc1ccc(O)cc1)NS(=O)(=O)c1ccccc1Cl |r| Show InChI InChI=1S/C22H29ClN4O3S/c1-15(2)11-18-13-25-22(24)27(18)14-17(12-16-7-9-19(28)10-8-16)26-31(29,30)21-6-4-3-5-20(21)23/h3-10,15,17-18,26,28H,11-14H2,1-2H3,(H2,24,25)/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
San Diego State University
Curated by ChEMBL
| Assay Description
Inhibition of LMTK3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127108
BindingDB Entry DOI: 10.7270/Q26M3BDB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase LMTK3
(Homo sapiens) | BDBM50542348

(CHEMBL4647760)Show SMILES Oc1ccc(C[C@@H](CN2[C@@H](CC3CCCCC3)CNC2=N)NS(=O)(=O)c2ccccc2Cl)cc1 |r| Show InChI InChI=1S/C25H33ClN4O3S/c26-23-8-4-5-9-24(23)34(32,33)29-20(14-19-10-12-22(31)13-11-19)17-30-21(16-28-25(30)27)15-18-6-2-1-3-7-18/h4-5,8-13,18,20-21,29,31H,1-3,6-7,14-17H2,(H2,27,28)/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
San Diego State University
Curated by ChEMBL
| Assay Description
Inhibition of LMTK3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127108
BindingDB Entry DOI: 10.7270/Q26M3BDB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase LMTK3
(Homo sapiens) | BDBM50542350
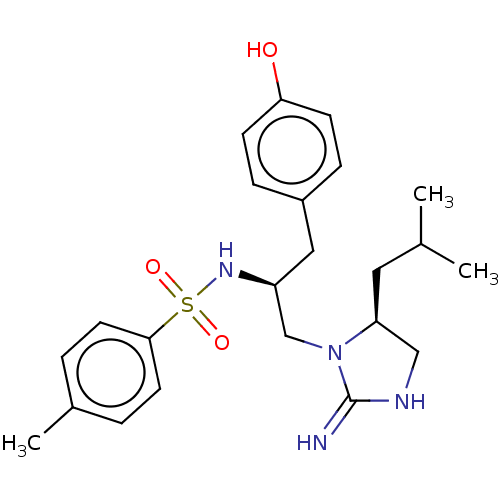
(CHEMBL4635320)Show SMILES CC(C)C[C@H]1CNC(=N)N1C[C@H](Cc1ccc(O)cc1)NS(=O)(=O)c1ccc(C)cc1 |r| Show InChI InChI=1S/C23H32N4O3S/c1-16(2)12-20-14-25-23(24)27(20)15-19(13-18-6-8-21(28)9-7-18)26-31(29,30)22-10-4-17(3)5-11-22/h4-11,16,19-20,26,28H,12-15H2,1-3H3,(H2,24,25)/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 308 | n/a | n/a | n/a | n/a | n/a | n/a |
San Diego State University
Curated by ChEMBL
| Assay Description
Inhibition of LMTK3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127108
BindingDB Entry DOI: 10.7270/Q26M3BDB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50614536

(CHEMBL5278149)Show SMILES Cc1cc(Oc2ccccc2)cc(C)c1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r,wD:18.19,(1.35,.46,;.58,1.79,;1.37,3.12,;.61,4.46,;1.39,5.78,;2.93,5.76,;3.72,7.09,;5.27,7.08,;6.02,5.73,;5.23,4.4,;3.69,4.43,;-.93,4.47,;-1.71,3.14,;-3.25,3.14,;-.96,1.81,;-1.65,.43,;-.93,-.93,;-2.01,-2.04,;-1.75,-3.56,;-2.85,-4.63,;-2.48,-6.12,;-1,-6.55,;.11,-5.48,;-.26,-3.98,;1.59,-5.89,;1.9,-7.09,;2.7,-4.83,;3.88,-5.17,;-3.38,-1.35,;-4.81,-1.92,;-6.02,-.97,;-5.8,.55,;-4.37,1.12,;-4.2,2.34,;-3.16,.17,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 314 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase LMTK3
(Homo sapiens) | BDBM50542349
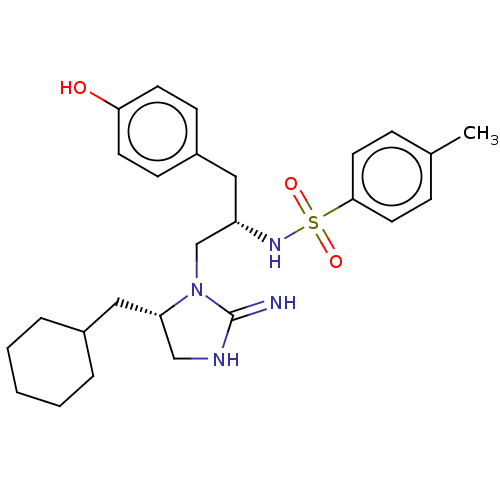
(CHEMBL4649625)Show SMILES Cc1ccc(cc1)S(=O)(=O)N[C@H](CN1[C@@H](CC2CCCCC2)CNC1=N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C26H36N4O3S/c1-19-7-13-25(14-8-19)34(32,33)29-22(15-21-9-11-24(31)12-10-21)18-30-23(17-28-26(30)27)16-20-5-3-2-4-6-20/h7-14,20,22-23,29,31H,2-6,15-18H2,1H3,(H2,27,28)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 318 | n/a | n/a | n/a | n/a | n/a | n/a |
San Diego State University
Curated by ChEMBL
| Assay Description
Inhibition of LMTK3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127108
BindingDB Entry DOI: 10.7270/Q26M3BDB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50614535

(CHEMBL5271284)Show SMILES Cc1cc(Oc2ccccc2)ccc1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 344 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase LMTK3
(Homo sapiens) | BDBM50542347
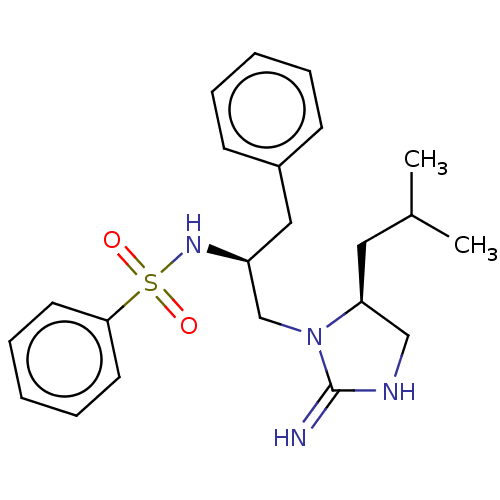
(CHEMBL4638119)Show SMILES CC(C)C[C@H]1CNC(=N)N1C[C@H](Cc1ccccc1)NS(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C22H30N4O2S/c1-17(2)13-20-15-24-22(23)26(20)16-19(14-18-9-5-3-6-10-18)25-29(27,28)21-11-7-4-8-12-21/h3-12,17,19-20,25H,13-16H2,1-2H3,(H2,23,24)/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 654 | n/a | n/a | n/a | n/a | n/a | n/a |
San Diego State University
Curated by ChEMBL
| Assay Description
Inhibition of LMTK3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127108
BindingDB Entry DOI: 10.7270/Q26M3BDB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50614535

(CHEMBL5271284)Show SMILES Cc1cc(Oc2ccccc2)ccc1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 874 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50447837
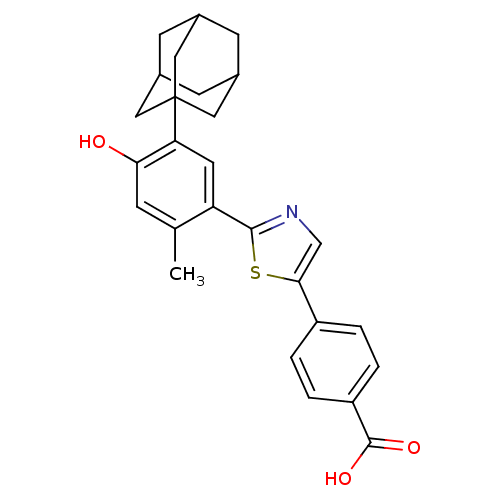
(CHEMBL3114297)Show SMILES Cc1cc(O)c(cc1-c1ncc(s1)-c1ccc(cc1)C(O)=O)C12CC3CC(CC(C3)C1)C2 |TLB:5:22:25:29.28.27,THB:23:24:27:31.22.30,23:22:25.24.29:27,30:22:25:29.28.27,30:28:25:31.23.22| Show InChI InChI=1S/C27H27NO3S/c1-15-6-23(29)22(27-11-16-7-17(12-27)9-18(8-16)13-27)10-21(15)25-28-14-24(32-25)19-2-4-20(5-3-19)26(30)31/h2-6,10,14,16-18,29H,7-9,11-13H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Antagonist activity at Gal4-fused RARalpha (unknown origin) transfected in HEK293 cells assessed as inhibition of ATRA-induced transcriptional activi... |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | |
Catechol O-methyltransferase
(Sus scrofa) | BDBM50004045

(2,3-Dihydroxy-4-nitro-benzaldehyde | CHEMBL357072)Show InChI InChI=1S/C7H5NO5/c9-3-4-1-2-5(8(12)13)7(11)6(4)10/h1-3,10-11H | MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Orgánica General (CSIC)
Curated by ChEMBL
| Assay Description
In vitro inhibition of Catechol-O-methyltransferase (COMT) from isolated partially purified pig liver enzyme |
J Med Chem 35: 4584-8 (1993)
BindingDB Entry DOI: 10.7270/Q2X63KWW |
More data for this
Ligand-Target Pair | |
Catechol O-methyltransferase
(Sus scrofa) | BDBM50004049

(3,4-Dihydroxy-2-nitro-benzaldehyde | CHEMBL139882)Show InChI InChI=1S/C7H5NO5/c9-3-4-1-2-5(10)7(11)6(4)8(12)13/h1-3,10-11H | MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Orgánica General (CSIC)
Curated by ChEMBL
| Assay Description
In vitro inhibition of Catechol-O-methyltransferase (COMT) from isolated partially purified pig liver enzyme |
J Med Chem 35: 4584-8 (1993)
BindingDB Entry DOI: 10.7270/Q2X63KWW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase LMTK3
(Homo sapiens) | BDBM50542346
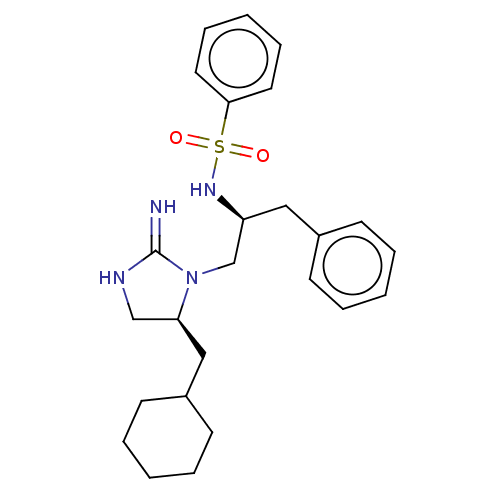
(CHEMBL4649046)Show SMILES N=C1NC[C@H](CC2CCCCC2)N1C[C@H](Cc1ccccc1)NS(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C25H34N4O2S/c26-25-27-18-23(17-21-12-6-2-7-13-21)29(25)19-22(16-20-10-4-1-5-11-20)28-32(30,31)24-14-8-3-9-15-24/h1,3-5,8-11,14-15,21-23,28H,2,6-7,12-13,16-19H2,(H2,26,27)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Diego State University
Curated by ChEMBL
| Assay Description
Inhibition of LMTK3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127108
BindingDB Entry DOI: 10.7270/Q26M3BDB |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50614536

(CHEMBL5278149)Show SMILES Cc1cc(Oc2ccccc2)cc(C)c1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r,wD:18.19,(1.35,.46,;.58,1.79,;1.37,3.12,;.61,4.46,;1.39,5.78,;2.93,5.76,;3.72,7.09,;5.27,7.08,;6.02,5.73,;5.23,4.4,;3.69,4.43,;-.93,4.47,;-1.71,3.14,;-3.25,3.14,;-.96,1.81,;-1.65,.43,;-.93,-.93,;-2.01,-2.04,;-1.75,-3.56,;-2.85,-4.63,;-2.48,-6.12,;-1,-6.55,;.11,-5.48,;-.26,-3.98,;1.59,-5.89,;1.9,-7.09,;2.7,-4.83,;3.88,-5.17,;-3.38,-1.35,;-4.81,-1.92,;-6.02,-.97,;-5.8,.55,;-4.37,1.12,;-4.2,2.34,;-3.16,.17,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Catechol O-methyltransferase
(Sus scrofa) | BDBM50004043
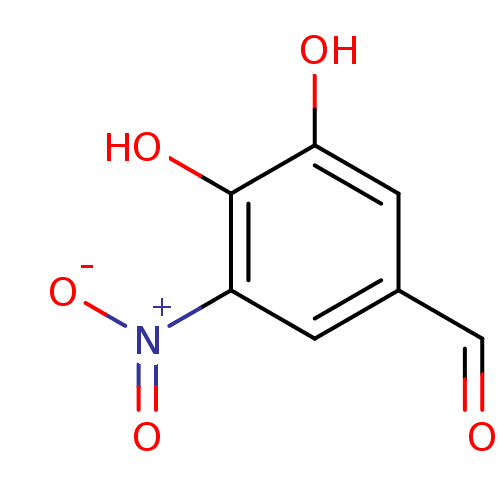
(3,4-Dihydroxy-5-Nitrobenzaldehyde (Rat) | 3,4-Dihy...)Show InChI InChI=1S/C7H5NO5/c9-3-4-1-5(8(12)13)7(11)6(10)2-4/h1-3,10-11H | MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Orgánica General (CSIC)
Curated by ChEMBL
| Assay Description
In vitro inhibition of Catechol-O-methyltransferase (COMT) from isolated partially purified pig liver enzyme |
J Med Chem 35: 4584-8 (1993)
BindingDB Entry DOI: 10.7270/Q2X63KWW |
More data for this
Ligand-Target Pair | |
Catechol O-methyltransferase
(Sus scrofa) | BDBM50004047

(2,3-Dihydroxy-5-nitro-benzaldehyde | CHEMBL141858)Show InChI InChI=1S/C7H5NO5/c9-3-4-1-5(8(12)13)2-6(10)7(4)11/h1-3,10-11H | MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Orgánica General (CSIC)
Curated by ChEMBL
| Assay Description
In vitro inhibition of Catechol-O-methyltransferase (COMT) from isolated partially purified pig liver enzyme |
J Med Chem 35: 4584-8 (1993)
BindingDB Entry DOI: 10.7270/Q2X63KWW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50614536

(CHEMBL5278149)Show SMILES Cc1cc(Oc2ccccc2)cc(C)c1-c1nn([C@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 |r,wD:18.19,(1.35,.46,;.58,1.79,;1.37,3.12,;.61,4.46,;1.39,5.78,;2.93,5.76,;3.72,7.09,;5.27,7.08,;6.02,5.73,;5.23,4.4,;3.69,4.43,;-.93,4.47,;-1.71,3.14,;-3.25,3.14,;-.96,1.81,;-1.65,.43,;-.93,-.93,;-2.01,-2.04,;-1.75,-3.56,;-2.85,-4.63,;-2.48,-6.12,;-1,-6.55,;.11,-5.48,;-.26,-3.98,;1.59,-5.89,;1.9,-7.09,;2.7,-4.83,;3.88,-5.17,;-3.38,-1.35,;-4.81,-1.92,;-6.02,-.97,;-5.8,.55,;-4.37,1.12,;-4.2,2.34,;-3.16,.17,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50447838
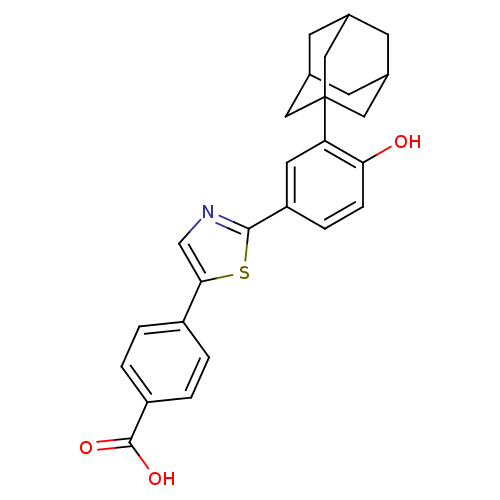
(CHEMBL3114296)Show SMILES OC(=O)c1ccc(cc1)-c1cnc(s1)-c1ccc(O)c(c1)C12CC3CC(CC(C3)C1)C2 |TLB:19:21:24:28.27.26,THB:22:23:26:30.21.29,22:21:24.23.28:26,29:21:24:28.27.26,29:27:24:30.22.21| Show InChI InChI=1S/C26H25NO3S/c28-22-6-5-20(10-21(22)26-11-15-7-16(12-26)9-17(8-15)13-26)24-27-14-23(31-24)18-1-3-19(4-2-18)25(29)30/h1-6,10,14-17,28H,7-9,11-13H2,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Antagonist activity at Gal4-fused RARalpha (unknown origin) transfected in HEK293 cells assessed as inhibition of ATRA-induced transcriptional activi... |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase LMTK3
(Homo sapiens) | BDBM50299148
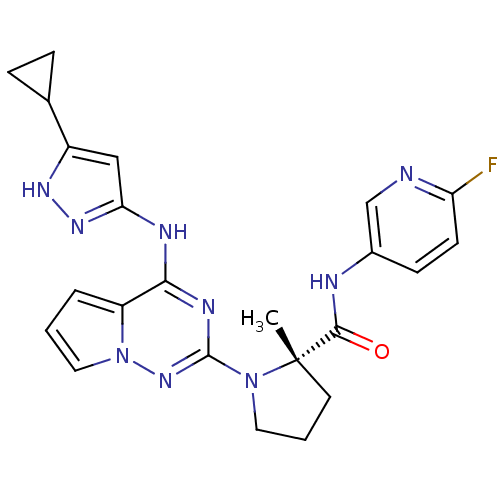
((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O/c1-23(21(34)26-15-7-8-18(24)25-13-15)9-3-10-32(23)22-28-20(17-4-2-11-33(17)31-22)27-19-12-16(29-30-19)14-5-6-14/h2,4,7-8,11-14H,3,5-6,9-10H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Diego State University
Curated by ChEMBL
| Assay Description
Inhibition of LMTK3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127108
BindingDB Entry DOI: 10.7270/Q26M3BDB |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50447838

(CHEMBL3114296)Show SMILES OC(=O)c1ccc(cc1)-c1cnc(s1)-c1ccc(O)c(c1)C12CC3CC(CC(C3)C1)C2 |TLB:19:21:24:28.27.26,THB:22:23:26:30.21.29,22:21:24.23.28:26,29:21:24:28.27.26,29:27:24:30.22.21| Show InChI InChI=1S/C26H25NO3S/c28-22-6-5-20(10-21(22)26-11-15-7-16(12-26)9-17(8-15)13-26)24-27-14-23(31-24)18-1-3-19(4-2-18)25(29)30/h1-6,10,14-17,28H,7-9,11-13H2,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Inhibition of recombinant IKKbeta (unknown origin) using ulight-IkappaomegaBalpha as substrate after 2 hrs by LANCE ultra TR-FRET assay |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50447835
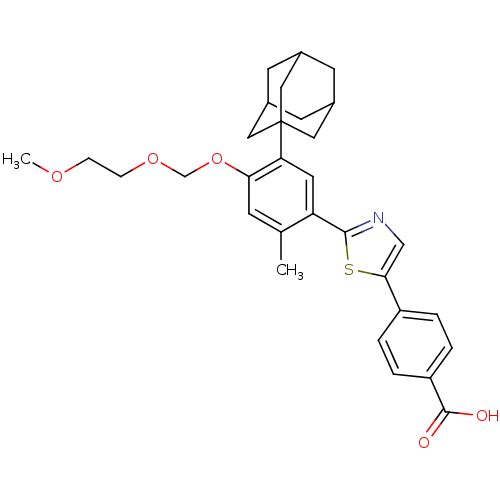
(CHEMBL3114299)Show SMILES COCCOCOc1cc(C)c(cc1C12CC3CC(CC(C3)C1)C2)-c1ncc(s1)-c1ccc(cc1)C(O)=O |TLB:13:14:17:21.20.19,THB:15:16:19:23.14.22,15:14:17.16.21:19,22:14:17:21.20.19,22:20:17:23.15.14| Show InChI InChI=1S/C31H35NO5S/c1-19-9-27(37-18-36-8-7-35-2)26(31-14-20-10-21(15-31)12-22(11-20)16-31)13-25(19)29-32-17-28(38-29)23-3-5-24(6-4-23)30(33)34/h3-6,9,13,17,20-22H,7-8,10-12,14-16,18H2,1-2H3,(H,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Inhibition of recombinant IKKbeta (unknown origin) using ulight-IkappaomegaBalpha as substrate after 2 hrs by LANCE ultra TR-FRET assay |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50244760

((E)-4-{3-[3-Adamantan-1-yl-4-(2-methoxy-ethoxymeth...)Show SMILES COCCOCOc1ccc(cc1C12CC3CC(CC(C3)C1)C2)C(=O)\C=C\c1ccc(cc1)C(O)=O |TLB:12:13:16:20.19.18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13| Show InChI InChI=1S/C30H34O6/c1-34-10-11-35-19-36-28-9-7-25(27(31)8-4-20-2-5-24(6-3-20)29(32)33)15-26(28)30-16-21-12-22(17-30)14-23(13-21)18-30/h2-9,15,21-23H,10-14,16-19H2,1H3,(H,32,33)/b8-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Antagonist activity at Gal4-fused RARalpha (unknown origin) transfected in HEK293 cells assessed as inhibition of ATRA-induced transcriptional activi... |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase LMTK3
(Homo sapiens) | BDBM158154

(US10081622, Compound 11 | US10370379, Entrectinib ...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCOCC2)c1 Show InChI InChI=1S/C31H34F2N6O2/c1-38-8-10-39(11-9-38)25-3-4-26(29(19-25)34-24-6-12-41-13-7-24)31(40)35-30-27-17-20(2-5-28(27)36-37-30)14-21-15-22(32)18-23(33)16-21/h2-5,15-19,24,34H,6-14H2,1H3,(H2,35,36,37,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
San Diego State University
Curated by ChEMBL
| Assay Description
Inhibition of LMTK3 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127108
BindingDB Entry DOI: 10.7270/Q26M3BDB |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50447839
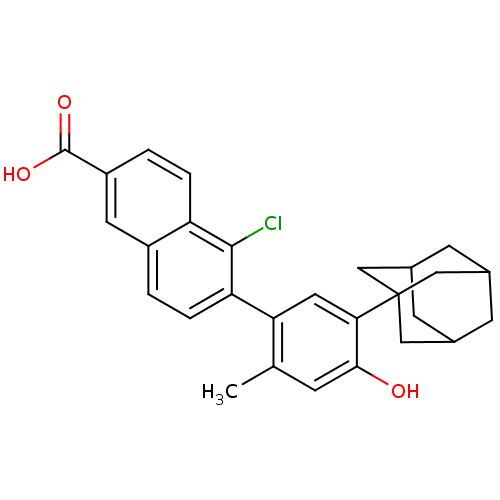
(CHEMBL565088)Show SMILES Cc1cc(O)c(cc1-c1ccc2cc(ccc2c1Cl)C(O)=O)C12CC3CC(CC(C3)C1)C2 |THB:27:26:23:29.28.30,27:28:23:25.31.26,30:22:25:29.27.28,30:28:25:23.22.31| Show InChI InChI=1S/C28H27ClO3/c1-15-6-25(30)24(28-12-16-7-17(13-28)9-18(8-16)14-28)11-23(15)22-5-2-19-10-20(27(31)32)3-4-21(19)26(22)29/h2-6,10-11,16-18,30H,7-9,12-14H2,1H3,(H,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Inhibition of recombinant IKKbeta (unknown origin) using ulight-IkappaomegaBalpha as substrate after 2 hrs by LANCE ultra TR-FRET assay |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50447837

(CHEMBL3114297)Show SMILES Cc1cc(O)c(cc1-c1ncc(s1)-c1ccc(cc1)C(O)=O)C12CC3CC(CC(C3)C1)C2 |TLB:5:22:25:29.28.27,THB:23:24:27:31.22.30,23:22:25.24.29:27,30:22:25:29.28.27,30:28:25:31.23.22| Show InChI InChI=1S/C27H27NO3S/c1-15-6-23(29)22(27-11-16-7-17(12-27)9-18(8-16)13-27)10-21(15)25-28-14-24(32-25)19-2-4-20(5-3-19)26(30)31/h2-6,10,14,16-18,29H,7-9,11-13H2,1H3,(H,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Inhibition of recombinant IKKbeta (unknown origin) using ulight-IkappaomegaBalpha as substrate after 2 hrs by LANCE ultra TR-FRET assay |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Mus musculus) | BDBM50235609
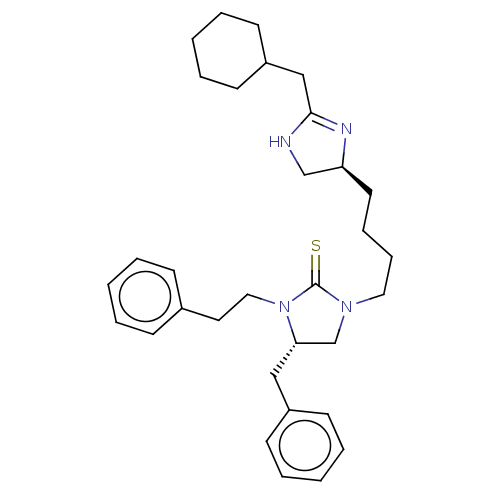
(CHEMBL4096280)Show SMILES S=C1N(CCCC[C@H]2CNC(CC3CCCCC3)=N2)C[C@H](Cc2ccccc2)N1CCc1ccccc1 |r,c:18| Show InChI InChI=1S/C32H44N4S/c37-32-35(20-11-10-18-29-24-33-31(34-29)23-28-16-8-3-9-17-28)25-30(22-27-14-6-2-7-15-27)36(32)21-19-26-12-4-1-5-13-26/h1-2,4-7,12-15,28-30H,3,8-11,16-25H2,(H,33,34)/t29-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse Gal4-fused DBD RORgamma LBD (Pro-261-end) expressed in TRex-CHOK1 cells after 20 hrs by luciferase reporter gene assay |
Bioorg Med Chem Lett 27: 1608-1610 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.014
BindingDB Entry DOI: 10.7270/Q29W0HRF |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50447836
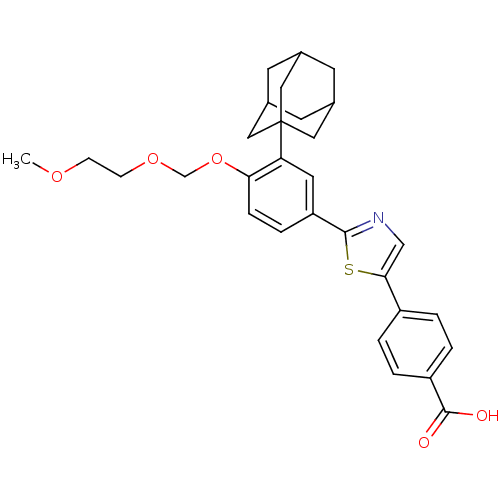
(CHEMBL3114298)Show SMILES COCCOCOc1ccc(cc1C12CC3CC(CC(C3)C1)C2)-c1ncc(s1)-c1ccc(cc1)C(O)=O |TLB:12:13:16:20.19.18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13| Show InChI InChI=1S/C30H33NO5S/c1-34-8-9-35-18-36-26-7-6-24(13-25(26)30-14-19-10-20(15-30)12-21(11-19)16-30)28-31-17-27(37-28)22-2-4-23(5-3-22)29(32)33/h2-7,13,17,19-21H,8-12,14-16,18H2,1H3,(H,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Vigo
Curated by ChEMBL
| Assay Description
Inhibition of recombinant IKKbeta (unknown origin) using ulight-IkappaomegaBalpha as substrate after 2 hrs by LANCE ultra TR-FRET assay |
Bioorg Med Chem 22: 1285-302 (2014)
Article DOI: 10.1016/j.bmc.2014.01.006
BindingDB Entry DOI: 10.7270/Q26W9CK1 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Mus musculus) | BDBM50235608
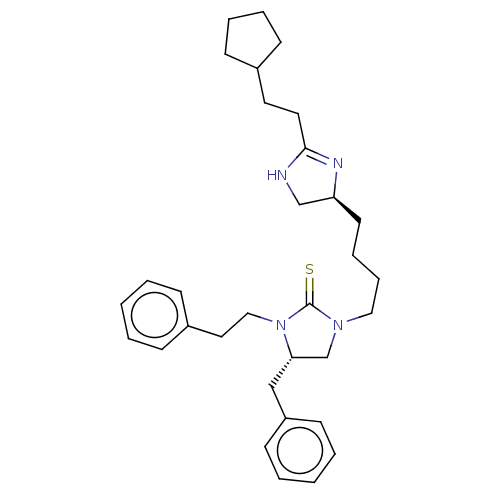
(CHEMBL4067306)Show SMILES S=C1N(CCCC[C@H]2CNC(CCC3CCCC3)=N2)C[C@H](Cc2ccccc2)N1CCc1ccccc1 |r,c:18| Show InChI InChI=1S/C32H44N4S/c37-32-35(21-10-9-17-29-24-33-31(34-29)19-18-26-13-7-8-14-26)25-30(23-28-15-5-2-6-16-28)36(32)22-20-27-11-3-1-4-12-27/h1-6,11-12,15-16,26,29-30H,7-10,13-14,17-25H2,(H,33,34)/t29-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse Gal4-fused DBD RORgamma LBD (Pro-261-end) expressed in TRex-CHOK1 cells after 20 hrs by luciferase reporter gene assay |
Bioorg Med Chem Lett 27: 1608-1610 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.014
BindingDB Entry DOI: 10.7270/Q29W0HRF |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Mus musculus) | BDBM50235604
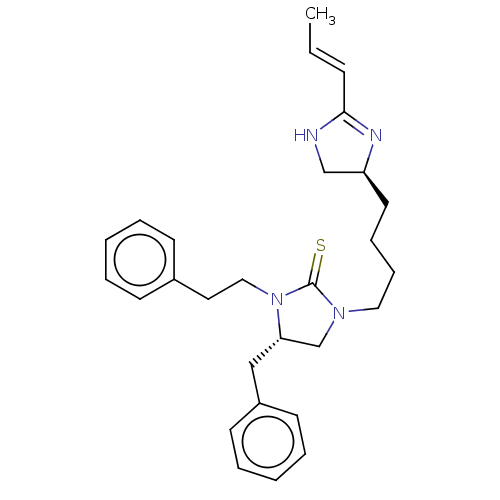
(CHEMBL4088559)Show SMILES C\C=C\C1=N[C@@H](CCCCN2C[C@H](Cc3ccccc3)N(CCc3ccccc3)C2=S)CN1 |r,t:3| Show InChI InChI=1S/C28H36N4S/c1-2-11-27-29-21-25(30-27)16-9-10-18-31-22-26(20-24-14-7-4-8-15-24)32(28(31)33)19-17-23-12-5-3-6-13-23/h2-8,11-15,25-26H,9-10,16-22H2,1H3,(H,29,30)/b11-2+/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse Gal4-fused DBD RORgamma LBD (Pro-261-end) expressed in TRex-CHOK1 cells after 20 hrs by luciferase reporter gene assay |
Bioorg Med Chem Lett 27: 1608-1610 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.014
BindingDB Entry DOI: 10.7270/Q29W0HRF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data