

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM69609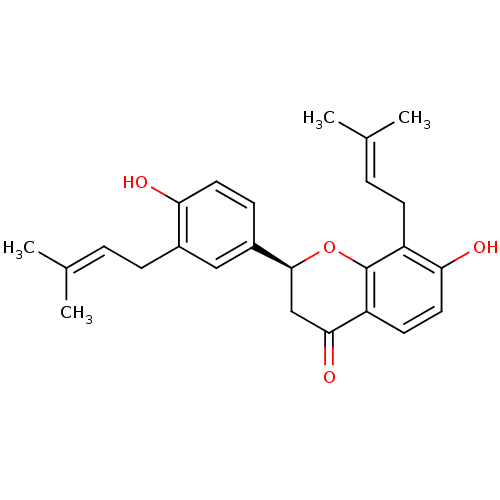 ((2S)-7-hydroxy-2-[4-hydroxy-3-(3-methylbut-2-enyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, People's Republic of China. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 25: 3706-3713 (2017) Article DOI: 10.1016/j.bmc.2017.05.009 BindingDB Entry DOI: 10.7270/Q2C82CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50253160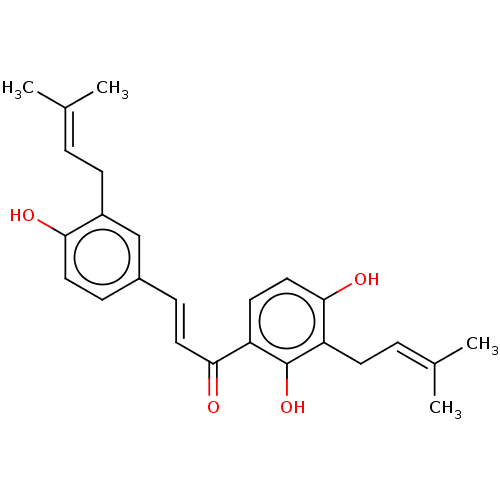 (Kanzonol C) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, People's Republic of China. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 25: 3706-3713 (2017) Article DOI: 10.1016/j.bmc.2017.05.009 BindingDB Entry DOI: 10.7270/Q2C82CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50253187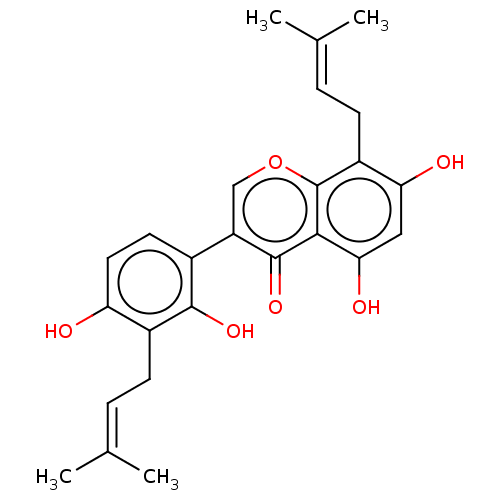 (CHEMBL3810262) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, People's Republic of China. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 25: 3706-3713 (2017) Article DOI: 10.1016/j.bmc.2017.05.009 BindingDB Entry DOI: 10.7270/Q2C82CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50253205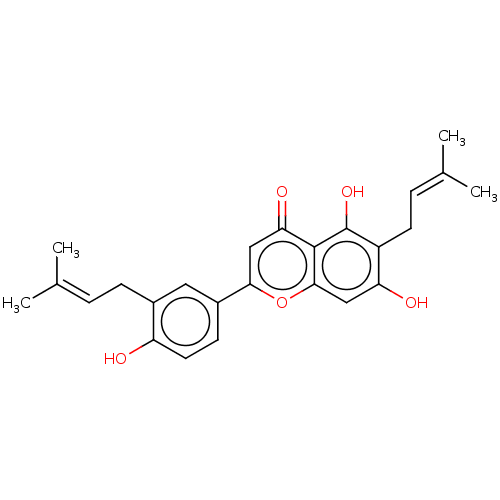 (CHEMBL4061417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, People's Republic of China. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 25: 3706-3713 (2017) Article DOI: 10.1016/j.bmc.2017.05.009 BindingDB Entry DOI: 10.7270/Q2C82CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50253206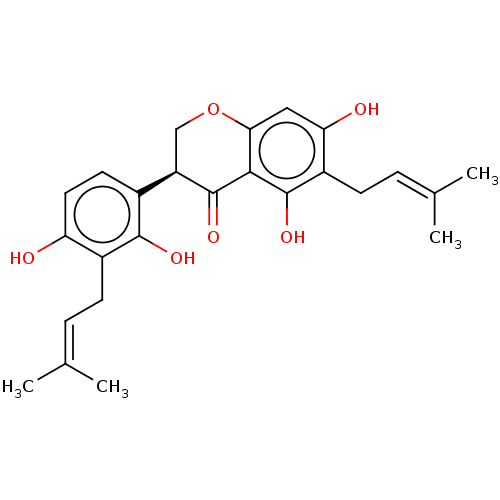 (CHEMBL4064802) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, People's Republic of China. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 25: 3706-3713 (2017) Article DOI: 10.1016/j.bmc.2017.05.009 BindingDB Entry DOI: 10.7270/Q2C82CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50212400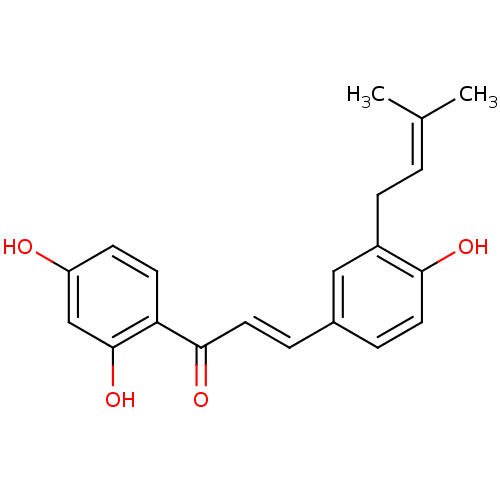 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, People's Republic of China. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 25: 3706-3713 (2017) Article DOI: 10.1016/j.bmc.2017.05.009 BindingDB Entry DOI: 10.7270/Q2C82CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50253207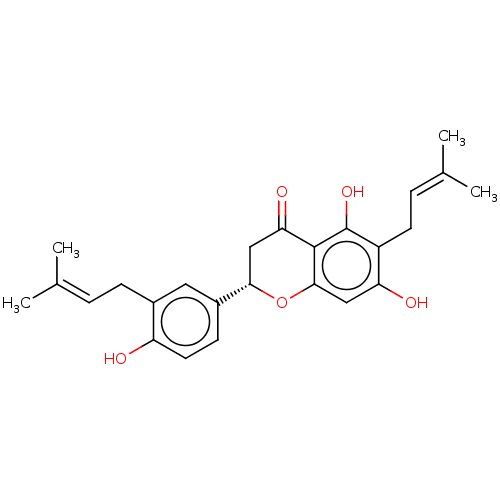 (MACARANGAFLAVANONE B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, People's Republic of China. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 25: 3706-3713 (2017) Article DOI: 10.1016/j.bmc.2017.05.009 BindingDB Entry DOI: 10.7270/Q2C82CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50253212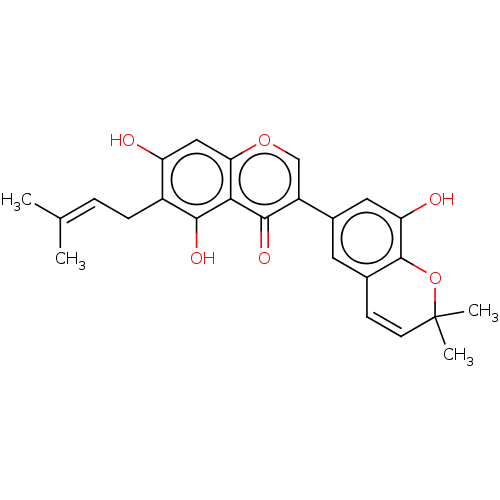 (CHEMBL4088099) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, People's Republic of China. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 25: 3706-3713 (2017) Article DOI: 10.1016/j.bmc.2017.05.009 BindingDB Entry DOI: 10.7270/Q2C82CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50253186 (CHEMBL4068000) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, People's Republic of China. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 25: 3706-3713 (2017) Article DOI: 10.1016/j.bmc.2017.05.009 BindingDB Entry DOI: 10.7270/Q2C82CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, People's Republic of China. Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 25: 3706-3713 (2017) Article DOI: 10.1016/j.bmc.2017.05.009 BindingDB Entry DOI: 10.7270/Q2C82CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||