

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol usi... | J Nat Prod 79: 899-906 (2016) Article DOI: 10.1021/acs.jnatprod.5b00952 BindingDB Entry DOI: 10.7270/Q23200DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50233538 (18beta-glycyrrhetic acid | 3beta-hydroxy-11-oxoole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol usi... | J Nat Prod 79: 899-906 (2016) Article DOI: 10.1021/acs.jnatprod.5b00952 BindingDB Entry DOI: 10.7270/Q23200DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498382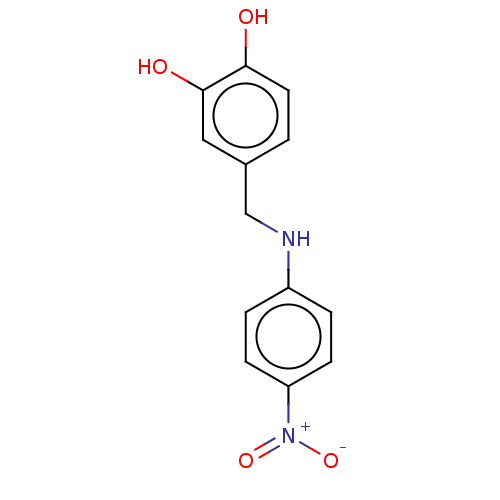 (CHEMBL3594250) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease isolated from Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol... | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50535797 (CHEMBL4532570) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol usi... | J Nat Prod 79: 899-906 (2016) Article DOI: 10.1021/acs.jnatprod.5b00952 BindingDB Entry DOI: 10.7270/Q23200DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50437699 (CHEMBL2409065) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol by ... | J Nat Prod 76: 1319-27 (2013) Article DOI: 10.1021/np400260g BindingDB Entry DOI: 10.7270/Q2JH3NMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM57506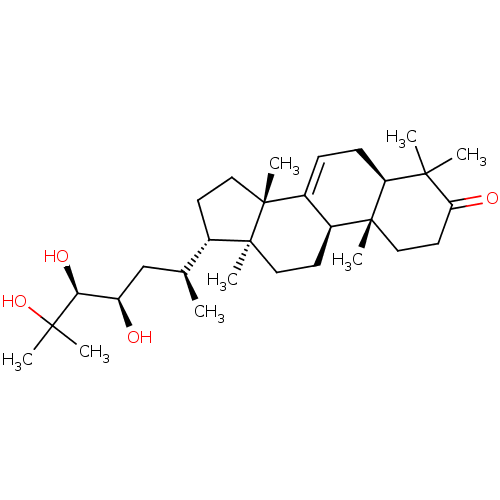 ((5R,9R,10R,13S,14S,17S)-4,4,10,13,14-pentamethyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol usi... | J Nat Prod 79: 899-906 (2016) Article DOI: 10.1021/acs.jnatprod.5b00952 BindingDB Entry DOI: 10.7270/Q23200DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50437698 (CHEMBL2409066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol by ... | J Nat Prod 76: 1319-27 (2013) Article DOI: 10.1021/np400260g BindingDB Entry DOI: 10.7270/Q2JH3NMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50535798 (CHEMBL4551399) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol usi... | J Nat Prod 79: 899-906 (2016) Article DOI: 10.1021/acs.jnatprod.5b00952 BindingDB Entry DOI: 10.7270/Q23200DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50535798 (CHEMBL4551399) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol usi... | J Nat Prod 79: 899-906 (2016) Article DOI: 10.1021/acs.jnatprod.5b00952 BindingDB Entry DOI: 10.7270/Q23200DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498382 (CHEMBL3594250) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol method | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498368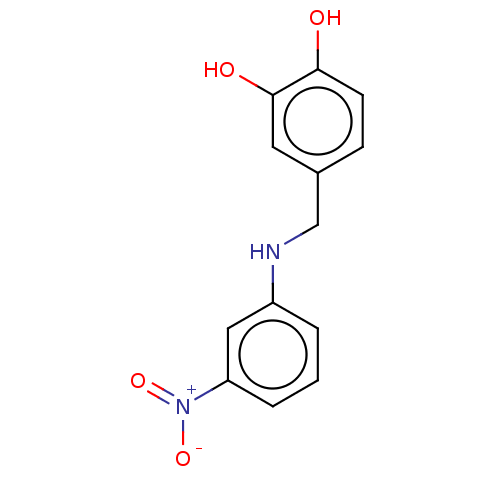 (CHEMBL3594254) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease isolated from Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol... | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50437699 (CHEMBL2409065) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol by ... | J Nat Prod 76: 1319-27 (2013) Article DOI: 10.1021/np400260g BindingDB Entry DOI: 10.7270/Q2JH3NMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50437698 (CHEMBL2409066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol by ... | J Nat Prod 76: 1319-27 (2013) Article DOI: 10.1021/np400260g BindingDB Entry DOI: 10.7270/Q2JH3NMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50535799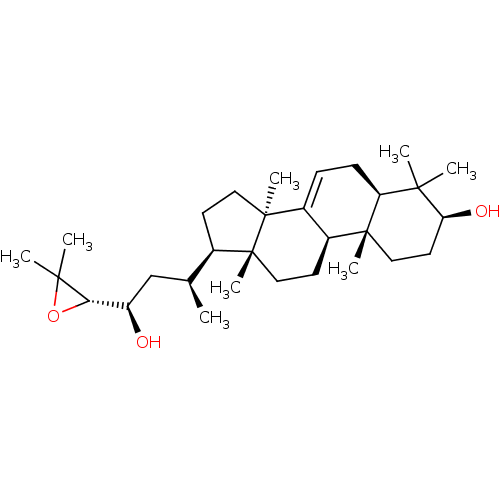 (CHEMBL4529492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol usi... | J Nat Prod 79: 899-906 (2016) Article DOI: 10.1021/acs.jnatprod.5b00952 BindingDB Entry DOI: 10.7270/Q23200DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531243 (CHEMBL4469442) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531243 (CHEMBL4469442) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531245 (CHEMBL4474641) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531245 (CHEMBL4474641) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498386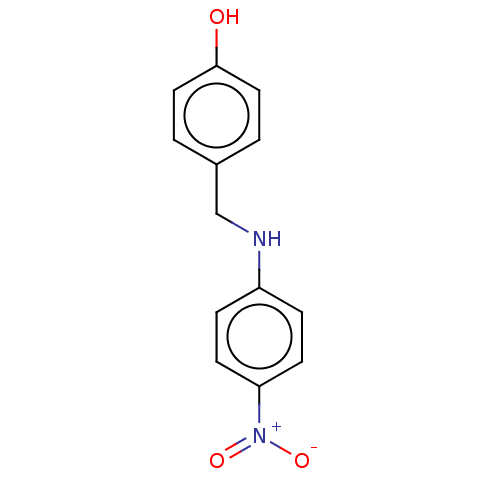 (CHEMBL3594251) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease isolated from Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol... | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531250 (CHEMBL4445427) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531250 (CHEMBL4445427) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498368 (CHEMBL3594254) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol method | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50535796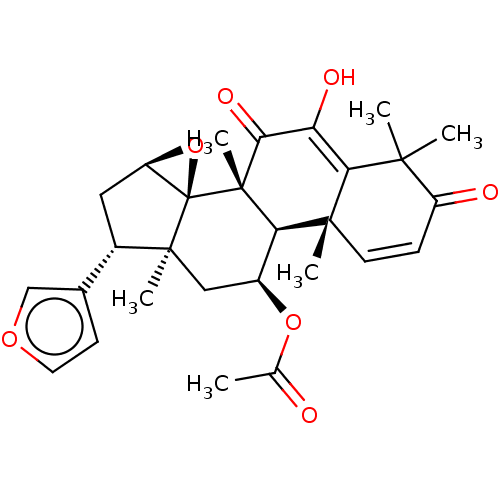 (CHEMBL4575439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol usi... | J Nat Prod 79: 899-906 (2016) Article DOI: 10.1021/acs.jnatprod.5b00952 BindingDB Entry DOI: 10.7270/Q23200DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531249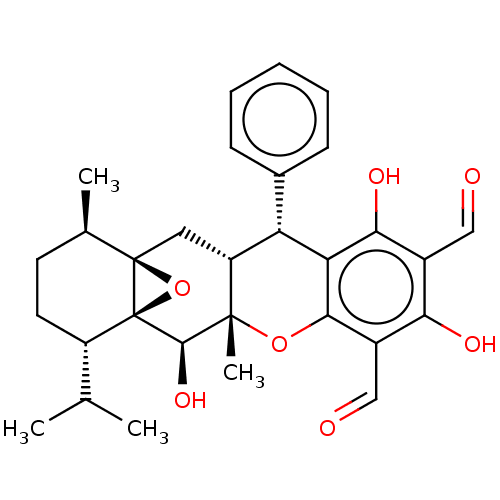 (CHEMBL4553740) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531249 (CHEMBL4553740) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531242 (CHEMBL4456002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531242 (CHEMBL4456002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50437700 (CHEMBL2409063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human microsomal 11beta-HSD1 overexpressed in HEK293 cells using [3H]-cortisone as substrate assessed as formation of [3H]-cortisol by ... | J Nat Prod 76: 1319-27 (2013) Article DOI: 10.1021/np400260g BindingDB Entry DOI: 10.7270/Q2JH3NMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531248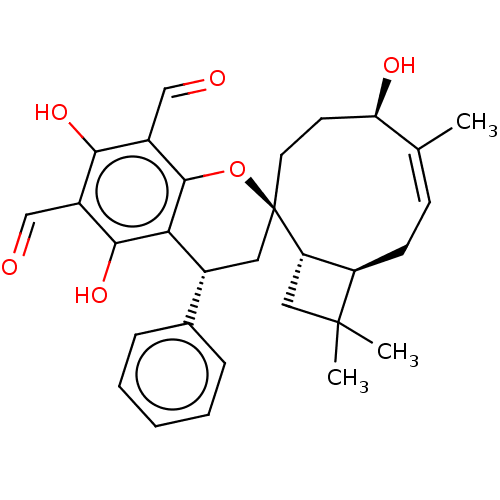 (CHEMBL4562591) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531248 (CHEMBL4562591) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498379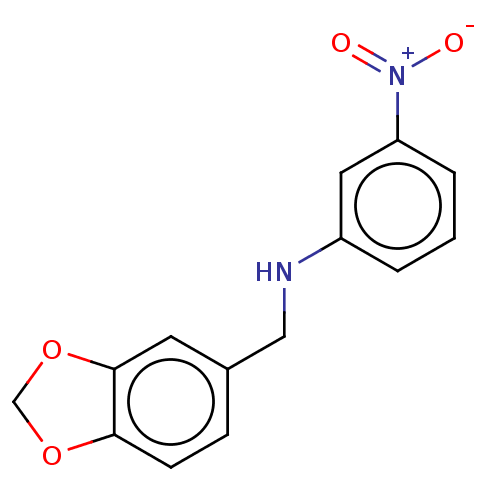 (CHEMBL3594256) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease isolated from Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol... | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498383 (CHEMBL3594257) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease isolated from Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol... | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50099857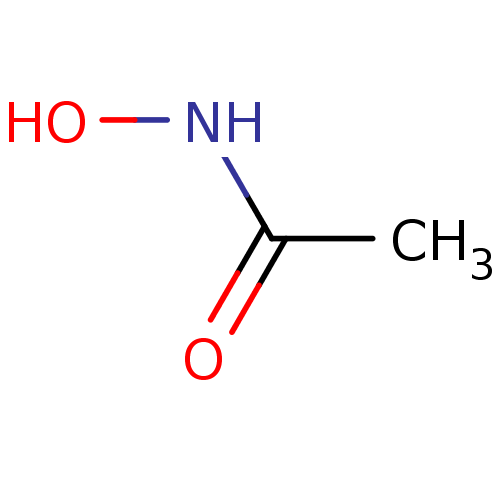 (ACETOHYDROXAMIC ACID (AHA) | AHA | Acethydroxamsae...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease isolated from Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol... | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531244 (CHEMBL4472595) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531244 (CHEMBL4472595) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531247 (CHEMBL4560923) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531247 (CHEMBL4560923) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498370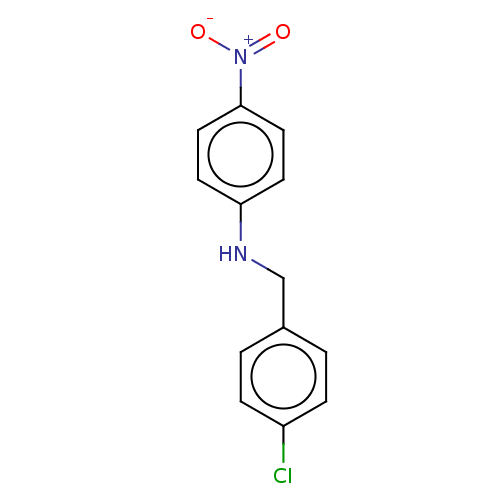 (CHEMBL3594252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease isolated from Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol... | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531251 (CHEMBL4448247) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531251 (CHEMBL4448247) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531246 (CHEMBL4454352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50531246 (CHEMBL4454352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498381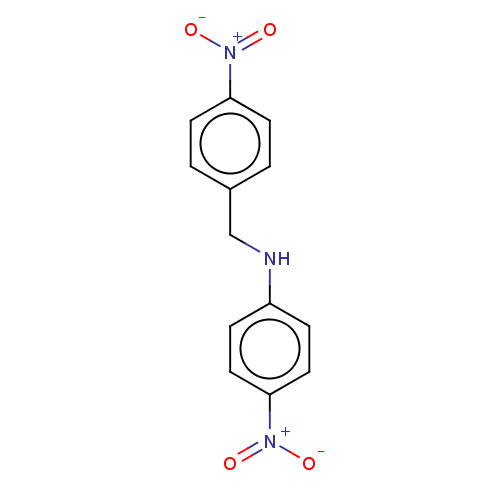 (CHEMBL3594253) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease isolated from Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol... | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498386 (CHEMBL3594251) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol method | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by ELISA | J Nat Prod 82: 3267-3278 (2019) Article DOI: 10.1021/acs.jnatprod.9b00333 BindingDB Entry DOI: 10.7270/Q2PG1W6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498385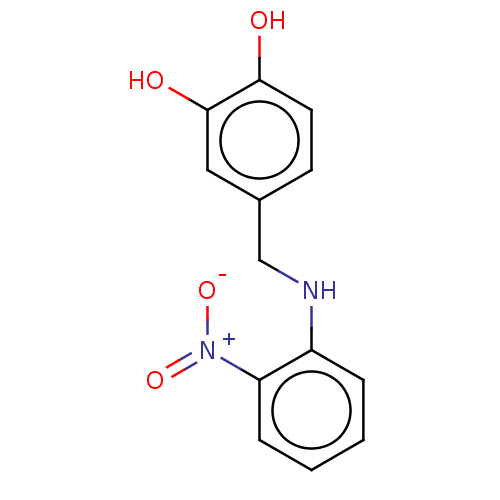 (CHEMBL3594261) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease isolated from Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol... | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498380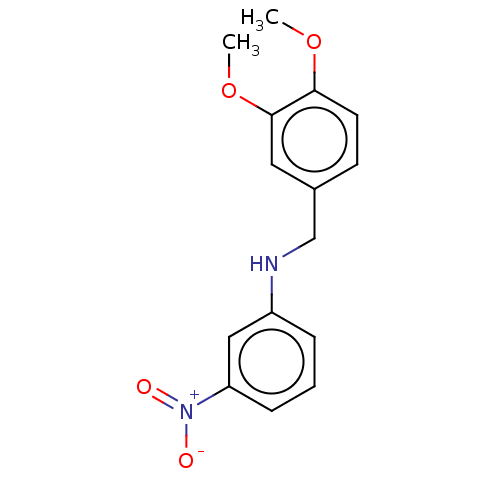 (CHEMBL3594255) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease isolated from Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol... | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498378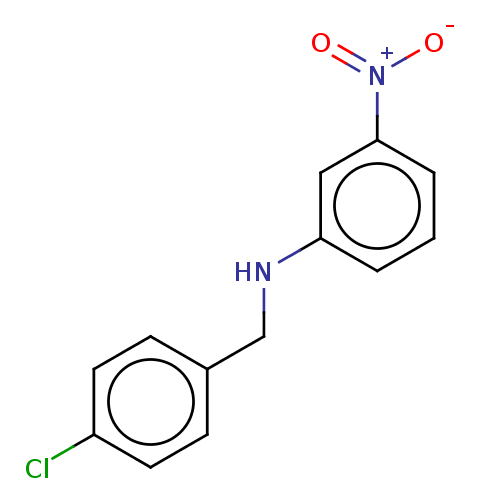 (CHEMBL3594258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease isolated from Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol... | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50498379 (CHEMBL3594256) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori ATCC 43504 assessed as ammonia production incubated for 1.5 hrs prior to testing by indophenol method | Bioorg Med Chem 23: 4508-4513 (2015) Article DOI: 10.1016/j.bmc.2015.06.014 BindingDB Entry DOI: 10.7270/Q2T43X33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 65 total ) | Next | Last >> |