

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012059 (CHEMBL3263302) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Displacement of ACPU from recombinant human soluble epoxide hydrolase after 1.5 hrs by FRET assay | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012016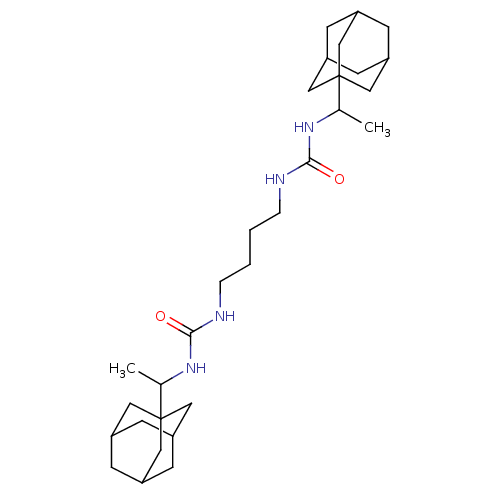 (CHEMBL3263294) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Displacement of ACPU from recombinant human soluble epoxide hydrolase after 1.5 hrs by FRET assay | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012054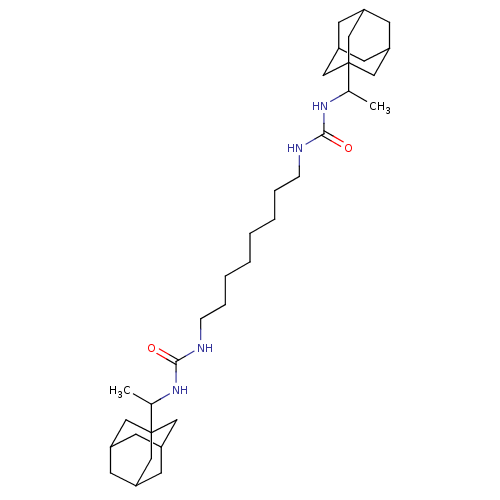 (CHEMBL3263297) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Displacement of ACPU from recombinant human soluble epoxide hydrolase after 1.5 hrs by FRET assay | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012011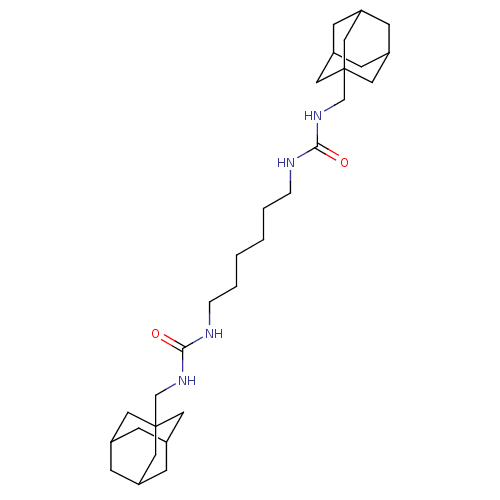 (CHEMBL3263289) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Displacement of ACPU from recombinant human soluble epoxide hydrolase after 1.5 hrs by FRET assay | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50546459 (CHEMBL4759033) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50546457 (CHEMBL4786305) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50546461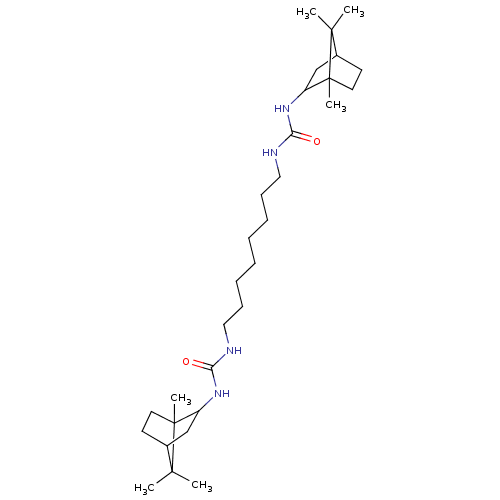 (CHEMBL4750771) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50546449 (CHEMBL4746902) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012011 (CHEMBL3263289) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539380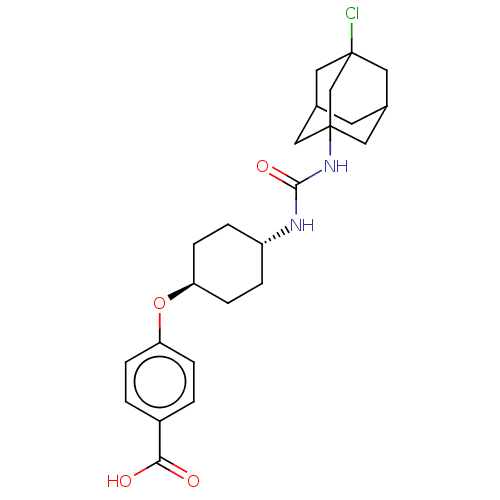 (CHEMBL4643551) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using cyano(2methoxy naphthalen-6-yl)methyl trans-(3-phenyloxyran-2-yl)methylcarbonate as substrate pre... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539356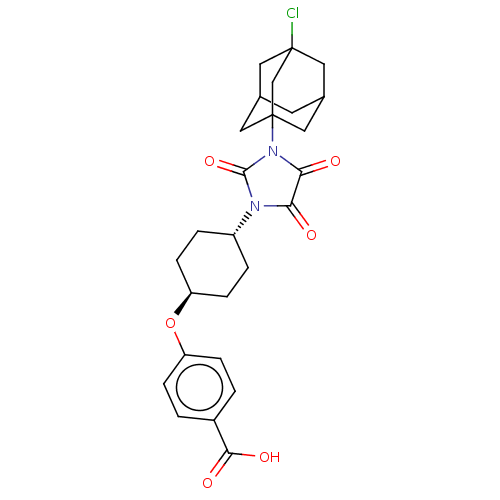 (CHEMBL4641404) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012054 (CHEMBL3263297) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50546453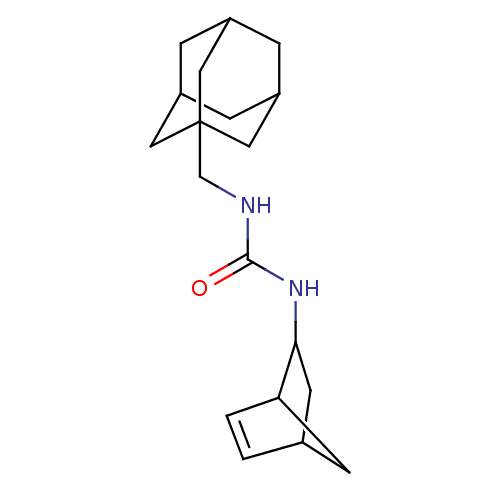 (CHEMBL4800466) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012011 (CHEMBL3263289) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539360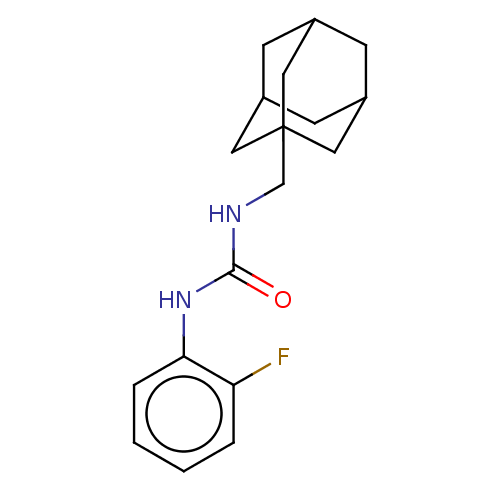 (CHEMBL4633887) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539384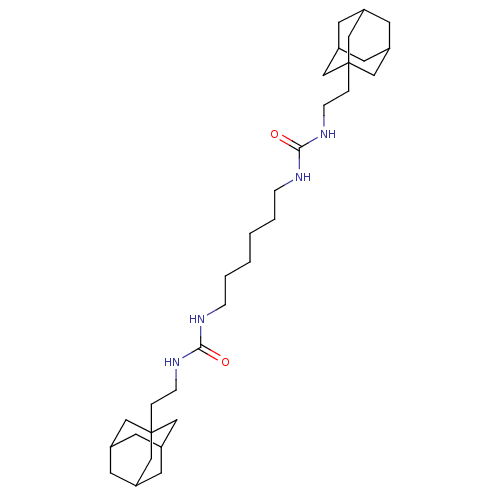 (CHEMBL4645669) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using cyano(2methoxy naphthalen-6-yl)methyl trans-(3-phenyloxyran-2-yl)methylcarbonate as substrate pre... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012054 (CHEMBL3263297) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50546454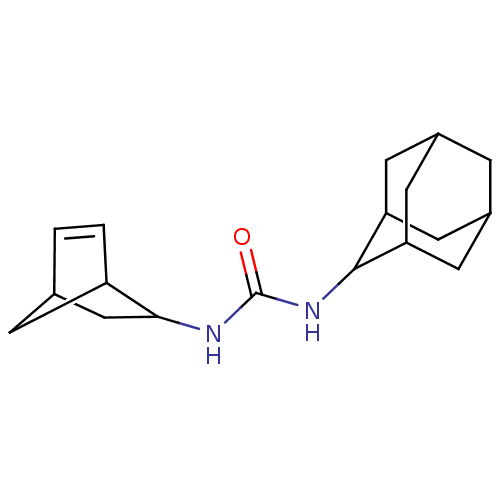 (CHEMBL4776297) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539358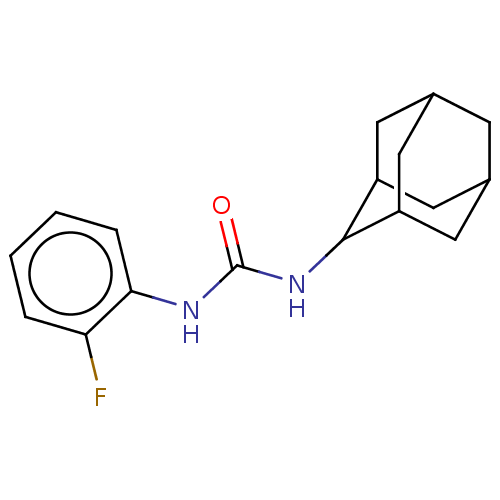 (CHEMBL4637039) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158481 (US9029401, 1728 (t-TUCB)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using CMNPC as substrate by fluorescence based assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM158481 (US9029401, 1728 (t-TUCB)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as fluorescent substrate | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012016 (CHEMBL3263294) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM129310 (US8815951, 411) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system using CMNPC as substrate preincubated for 5 mins followed by substrate... | Bioorg Med Chem Lett 28: 2302-2313 (2018) Article DOI: 10.1016/j.bmcl.2018.05.024 BindingDB Entry DOI: 10.7270/Q2222X8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM129310 (US8815951, 411) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM129310 (US8815951, 411) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50546447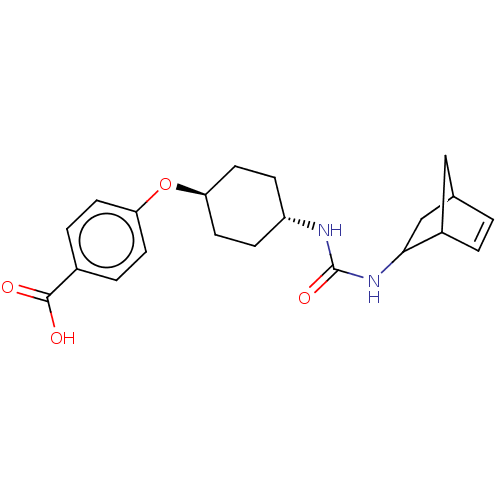 (CHEMBL4755752) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539385 (CHEMBL4641883) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using cyano(2methoxy naphthalen-6-yl)methyl trans-(3-phenyloxyran-2-yl)methylcarbonate as substrate pre... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539371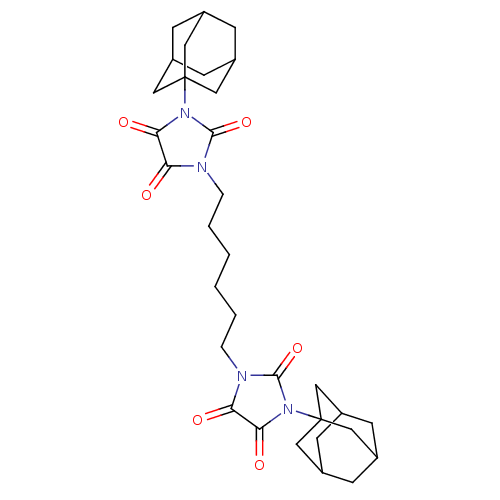 (CHEMBL4642121) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012017 (CHEMBL3263295) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50546460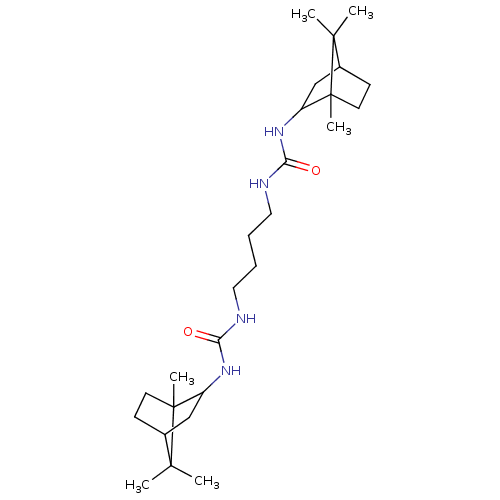 (CHEMBL4762498) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50546450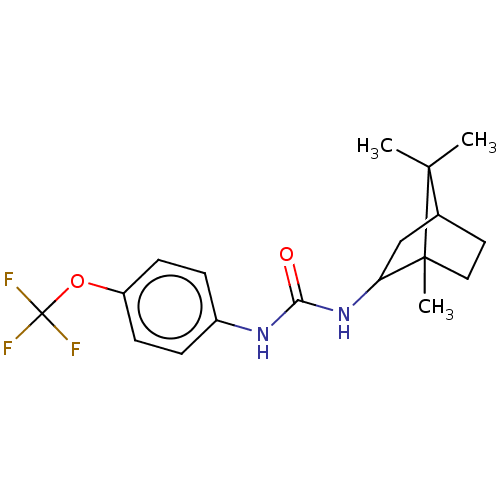 (CHEMBL4790175) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012016 (CHEMBL3263294) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012059 (CHEMBL3263302) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM25731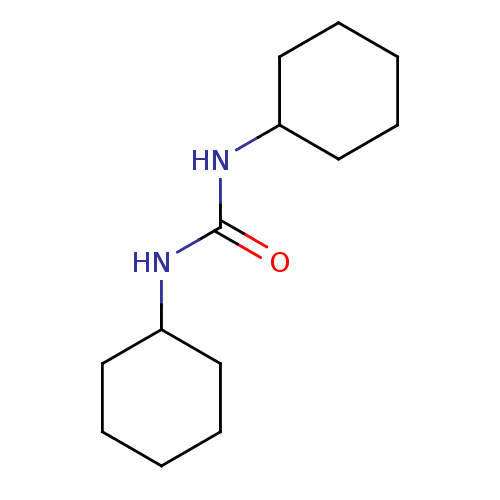 (1,3-dicyclohexylurea | CHEMBL1458 | US8815951, 1,3...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539383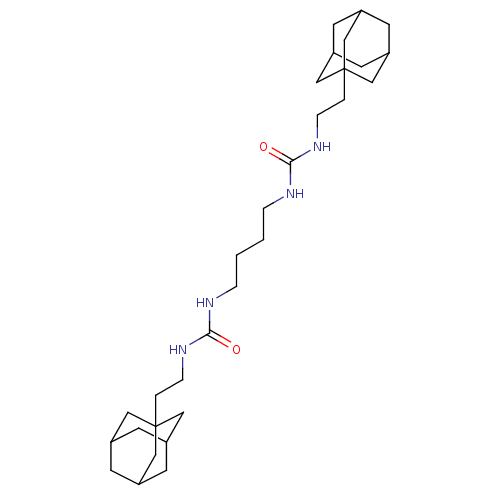 (CHEMBL4644608) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using cyano(2methoxy naphthalen-6-yl)methyl trans-(3-phenyloxyran-2-yl)methylcarbonate as substrate pre... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539364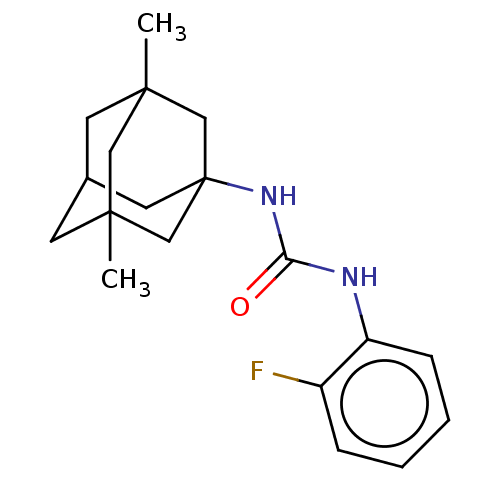 (CHEMBL4649528) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50546441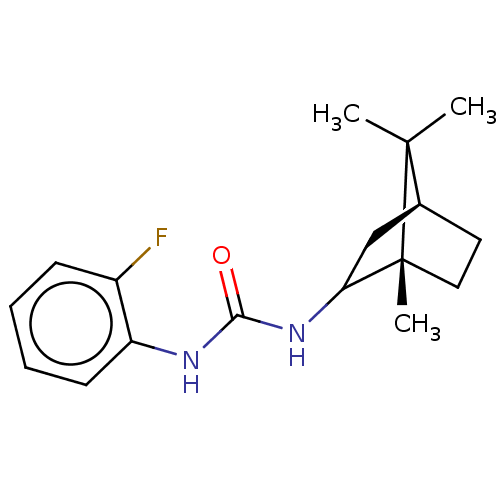 (CHEMBL4778603) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539372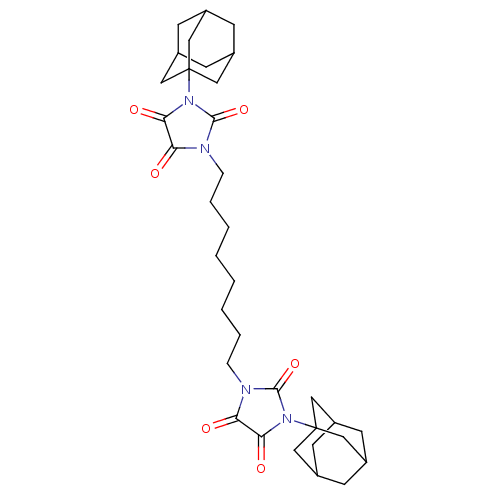 (CHEMBL4644920) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012059 (CHEMBL3263302) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133707 (CHEMBL3633681) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539354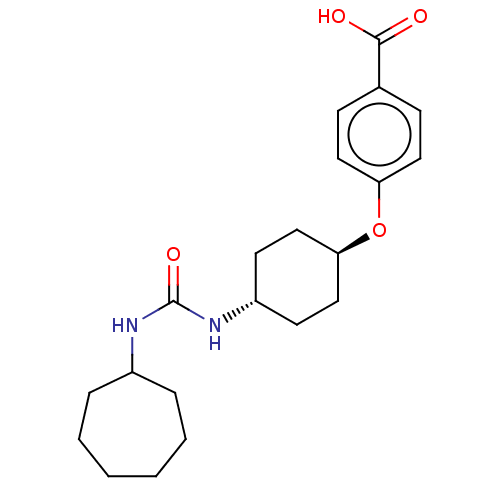 (CHEMBL4642519) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133694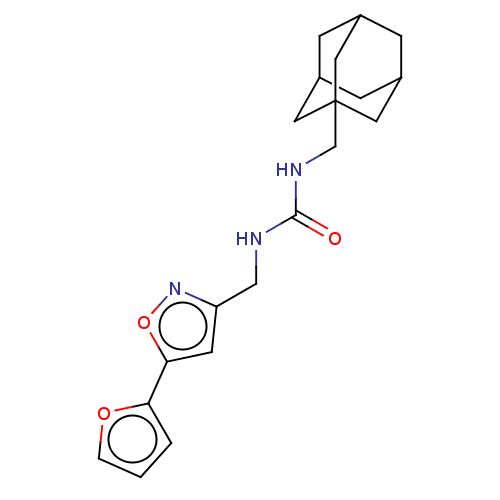 (CHEMBL3633668) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM25731 (1,3-dicyclohexylurea | CHEMBL1458 | US8815951, 1,3...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50546452 (CHEMBL4799688) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539379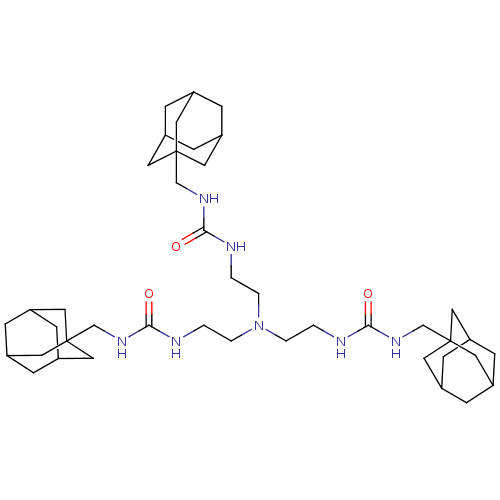 (CHEMBL4646845) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126908 BindingDB Entry DOI: 10.7270/Q2M0490F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50133695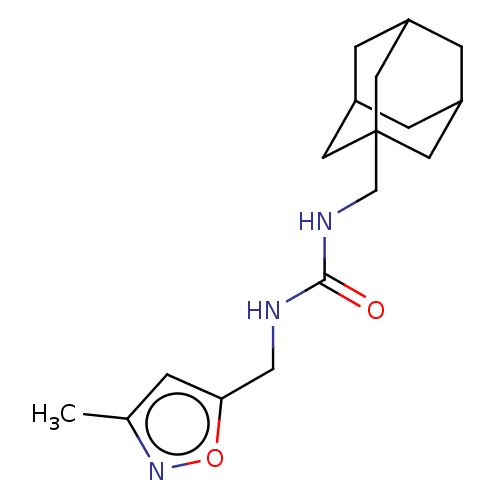 (CHEMBL3633669) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate by kinetic fluorescent assay | Bioorg Med Chem Lett 25: 5514-9 (2015) Article DOI: 10.1016/j.bmcl.2015.10.066 BindingDB Entry DOI: 10.7270/Q28P62C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50546445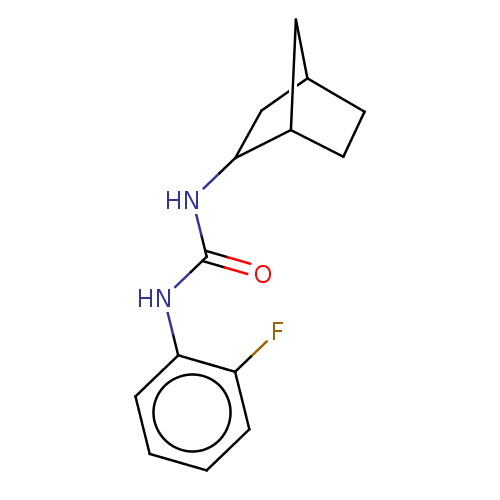 (CHEMBL4748031) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127430 BindingDB Entry DOI: 10.7270/Q2WQ07D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50274193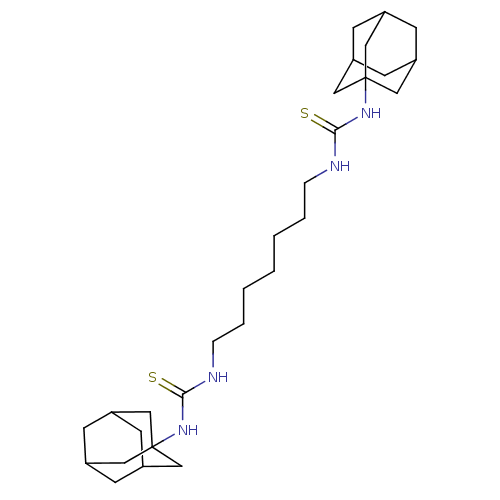 (CHEMBL4128873) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of recombinant human sEH expressed in baculovirus expression system using CMNPC as substrate preincubated for 5 mins followed by substrate... | Bioorg Med Chem Lett 28: 2302-2313 (2018) Article DOI: 10.1016/j.bmcl.2018.05.024 BindingDB Entry DOI: 10.7270/Q2222X8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012015 (CHEMBL3263293) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50012012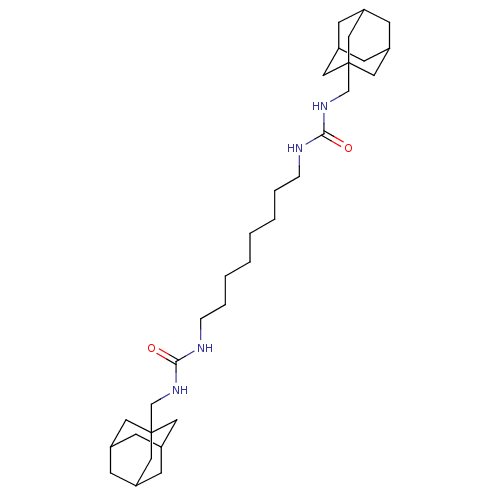 (CHEMBL3263290) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Volzhsky Polytechnic Institute (branch) Volgograd State Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition measured at... | Bioorg Med Chem Lett 24: 2193-7 (2014) Article DOI: 10.1016/j.bmcl.2014.03.016 BindingDB Entry DOI: 10.7270/Q2PR7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 181 total ) | Next | Last >> |