 Found 243 hits with Last Name = 'chen' and Initial = 'gr'
Found 243 hits with Last Name = 'chen' and Initial = 'gr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50347243
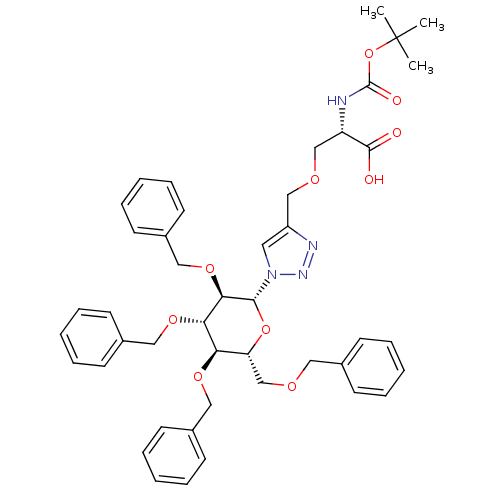
(CHEMBL1795950)Show SMILES CC(C)(C)OC(=O)N[C@@H](COCc1cn(nn1)[C@@H]1O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C45H52N4O10/c1-45(2,3)59-44(52)46-37(43(50)51)30-54-29-36-24-49(48-47-36)42-41(57-28-35-22-14-7-15-23-35)40(56-27-34-20-12-6-13-21-34)39(55-26-33-18-10-5-11-19-33)38(58-42)31-53-25-32-16-8-4-9-17-32/h4-24,37-42H,25-31H2,1-3H3,(H,46,52)(H,50,51)/t37-,38+,39+,40-,41+,42+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.5 | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant PTB1B catalytic domain using pNPP substrate at 30 degC and pH 6.5 by Eadie-Hofstee plot |
Bioorg Med Chem 19: 3892-900 (2011)
Article DOI: 10.1016/j.bmc.2011.05.049
BindingDB Entry DOI: 10.7270/Q2RJ4JTV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50273794
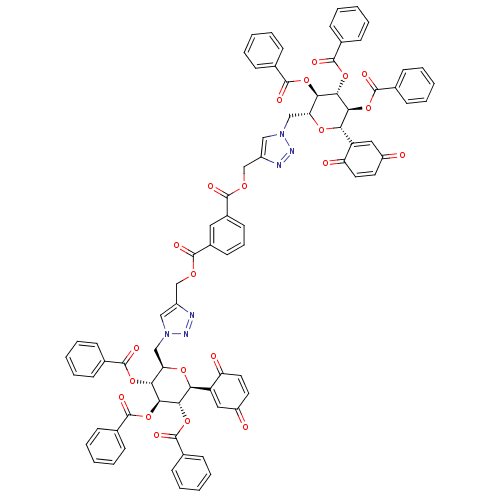
(CHEMBL446857 | bis((1-(((2R,3R,4R,5S,6S)-3,4,5-tri...)Show SMILES O=C(OCc1cn(C[C@H]2O[C@H]([C@H](OC(=O)c3ccccc3)[C@@H](OC(=O)c3ccccc3)[C@@H]2OC(=O)c2ccccc2)C2=CC(=O)C=CC2=O)nn1)c1cccc(c1)C(=O)OCc1cn(C[C@H]2O[C@H]([C@H](OC(=O)c3ccccc3)[C@@H](OC(=O)c3ccccc3)[C@@H]2OC(=O)c2ccccc2)C2=CC(=O)C=CC2=O)nn1 |r,c:49,113,t:45,109| Show InChI InChI=1S/C80H60N6O22/c87-57-34-36-61(89)59(39-57)65-69(105-77(95)49-24-11-3-12-25-49)71(107-79(97)51-28-15-5-16-29-51)67(103-75(93)47-20-7-1-8-21-47)63(101-65)43-85-41-55(81-83-85)45-99-73(91)53-32-19-33-54(38-53)74(92)100-46-56-42-86(84-82-56)44-64-68(104-76(94)48-22-9-2-10-23-48)72(108-80(98)52-30-17-6-18-31-52)70(106-78(96)50-26-13-4-14-27-50)66(102-64)60-40-58(88)35-37-62(60)90/h1-42,63-72H,43-46H2/t63-,64-,65+,66+,67-,68-,69+,70+,71+,72+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B catalytic domain |
Bioorg Med Chem 16: 9757-63 (2008)
Article DOI: 10.1016/j.bmc.2008.09.066
BindingDB Entry DOI: 10.7270/Q2G73DJN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50273795
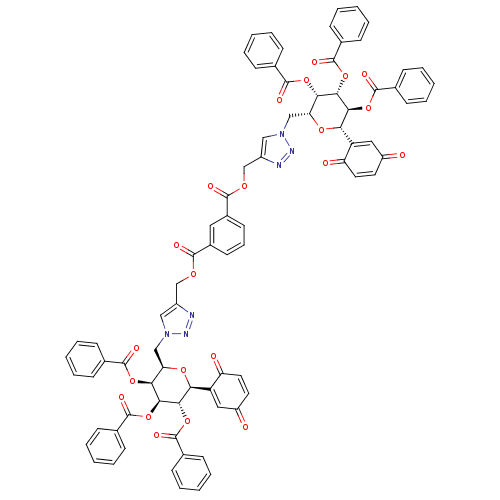
(CHEMBL510405 | bis((1-(((2R,3S,4R,5S,6S)-3,4,5-tri...)Show SMILES O=C(OCc1cn(C[C@H]2O[C@H]([C@H](OC(=O)c3ccccc3)[C@@H](OC(=O)c3ccccc3)[C@H]2OC(=O)c2ccccc2)C2=CC(=O)C=CC2=O)nn1)c1cccc(c1)C(=O)OCc1cn(C[C@H]2O[C@H]([C@H](OC(=O)c3ccccc3)[C@@H](OC(=O)c3ccccc3)[C@H]2OC(=O)c2ccccc2)C2=CC(=O)C=CC2=O)nn1 |r,c:49,113,t:45,109| Show InChI InChI=1S/C80H60N6O22/c87-57-34-36-61(89)59(39-57)65-69(105-77(95)49-24-11-3-12-25-49)71(107-79(97)51-28-15-5-16-29-51)67(103-75(93)47-20-7-1-8-21-47)63(101-65)43-85-41-55(81-83-85)45-99-73(91)53-32-19-33-54(38-53)74(92)100-46-56-42-86(84-82-56)44-64-68(104-76(94)48-22-9-2-10-23-48)72(108-80(98)52-30-17-6-18-31-52)70(106-78(96)50-26-13-4-14-27-50)66(102-64)60-40-58(88)35-37-62(60)90/h1-42,63-72H,43-46H2/t63-,64-,65+,66+,67+,68+,69+,70+,71+,72+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B catalytic domain |
Bioorg Med Chem 16: 9757-63 (2008)
Article DOI: 10.1016/j.bmc.2008.09.066
BindingDB Entry DOI: 10.7270/Q2G73DJN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50245900
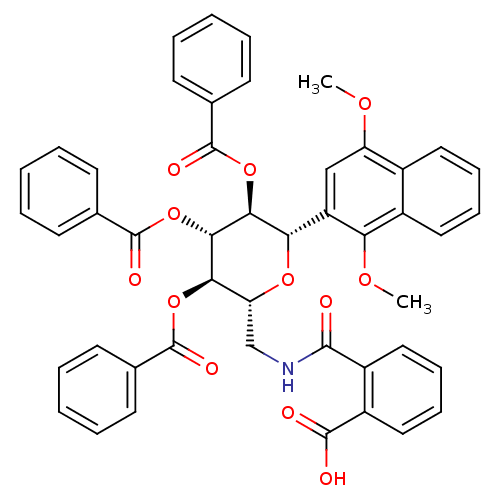
(2-((((2R,3R,4R,5S,6S)-3,4,5-tris(benzoyloxy)-6-(1,...)Show SMILES COc1cc([C@@H]2O[C@H](CNC(=O)c3ccccc3C(O)=O)[C@@H](OC(=O)c3ccccc3)[C@H](OC(=O)c3ccccc3)[C@H]2OC(=O)c2ccccc2)c(OC)c2ccccc12 |r| Show InChI InChI=1S/C47H39NO12/c1-55-36-26-35(38(56-2)32-23-13-12-22-31(32)36)39-41(59-46(53)29-18-8-4-9-19-29)42(60-47(54)30-20-10-5-11-21-30)40(58-45(52)28-16-6-3-7-17-28)37(57-39)27-48-43(49)33-24-14-15-25-34(33)44(50)51/h3-26,37,39-42H,27H2,1-2H3,(H,48,49)(H,50,51)/t37-,39+,40-,41+,42+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PTP1B |
Bioorg Med Chem Lett 18: 6348-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.091
BindingDB Entry DOI: 10.7270/Q28W3F68 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50273792

(CHEMBL505561 | bis((1-(((2R,3R,4R,5S,6S)-3,4,5-tri...)Show SMILES COc1ccc(OC)c(c1)[C@@H]1O[C@H](Cn2cc(COC(=O)c3cccc(c3)C(=O)OCc3cn(C[C@H]4O[C@H]([C@H](OC(=O)c5ccccc5)[C@@H](OC(=O)c5ccccc5)[C@@H]4OC(=O)c4ccccc4)c4cc(OC)ccc4OC)nn3)nn2)[C@@H](OC(=O)c2ccccc2)[C@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1 |r| Show InChI InChI=1S/C84H72N6O22/c1-99-61-38-40-65(101-3)63(43-61)69-73(109-81(95)53-28-15-7-16-29-53)75(111-83(97)55-32-19-9-20-33-55)71(107-79(93)51-24-11-5-12-25-51)67(105-69)47-89-45-59(85-87-89)49-103-77(91)57-36-23-37-58(42-57)78(92)104-50-60-46-90(88-86-60)48-68-72(108-80(94)52-26-13-6-14-27-52)76(112-84(98)56-34-21-10-22-35-56)74(110-82(96)54-30-17-8-18-31-54)70(106-68)64-44-62(100-2)39-41-66(64)102-4/h5-46,67-76H,47-50H2,1-4H3/t67-,68-,69+,70+,71-,72-,73+,74+,75+,76+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B catalytic domain |
Bioorg Med Chem 16: 9757-63 (2008)
Article DOI: 10.1016/j.bmc.2008.09.066
BindingDB Entry DOI: 10.7270/Q2G73DJN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50273793
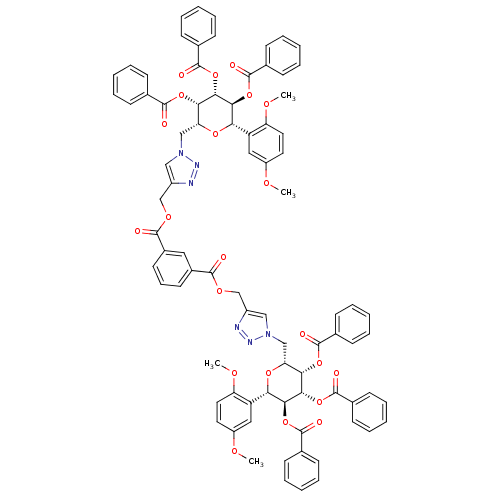
(CHEMBL508150 | bis((1-(((2R,3S,4R,5S,6S)-3,4,5-tri...)Show SMILES COc1ccc(OC)c(c1)[C@@H]1O[C@H](Cn2cc(COC(=O)c3cccc(c3)C(=O)OCc3cn(C[C@H]4O[C@H]([C@H](OC(=O)c5ccccc5)[C@@H](OC(=O)c5ccccc5)[C@H]4OC(=O)c4ccccc4)c4cc(OC)ccc4OC)nn3)nn2)[C@H](OC(=O)c2ccccc2)[C@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1 |r| Show InChI InChI=1S/C84H72N6O22/c1-99-61-38-40-65(101-3)63(43-61)69-73(109-81(95)53-28-15-7-16-29-53)75(111-83(97)55-32-19-9-20-33-55)71(107-79(93)51-24-11-5-12-25-51)67(105-69)47-89-45-59(85-87-89)49-103-77(91)57-36-23-37-58(42-57)78(92)104-50-60-46-90(88-86-60)48-68-72(108-80(94)52-26-13-6-14-27-52)76(112-84(98)56-34-21-10-22-35-56)74(110-82(96)54-30-17-8-18-31-54)70(106-68)64-44-62(100-2)39-41-66(64)102-4/h5-46,67-76H,47-50H2,1-4H3/t67-,68-,69+,70+,71+,72+,73+,74+,75+,76+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B catalytic domain |
Bioorg Med Chem 16: 9757-63 (2008)
Article DOI: 10.1016/j.bmc.2008.09.066
BindingDB Entry DOI: 10.7270/Q2G73DJN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50245897
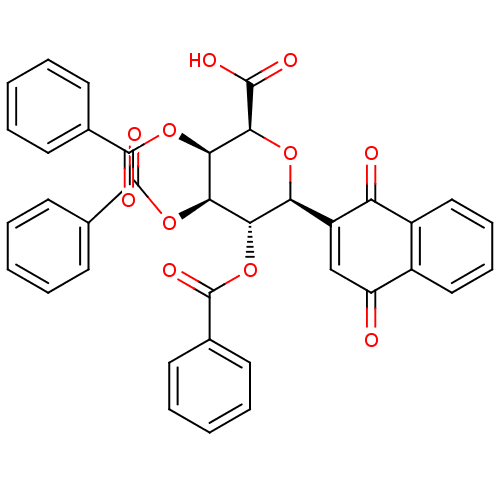
((2S,3R,4R,5S,6S)-3,4,5-tris(benzoyloxy)-6-(1,4-dio...)Show SMILES OC(=O)[C@H]1O[C@H]([C@H](OC(=O)c2ccccc2)[C@@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1)C1=CC(=O)c2ccccc2C1=O |r,t:40| Show InChI InChI=1S/C37H26O11/c38-27-20-26(28(39)25-19-11-10-18-24(25)27)29-30(46-35(42)21-12-4-1-5-13-21)31(47-36(43)22-14-6-2-7-15-22)32(33(45-29)34(40)41)48-37(44)23-16-8-3-9-17-23/h1-20,29-33H,(H,40,41)/t29-,30-,31+,32+,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PTP1B |
Bioorg Med Chem Lett 18: 6348-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.091
BindingDB Entry DOI: 10.7270/Q28W3F68 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM125938
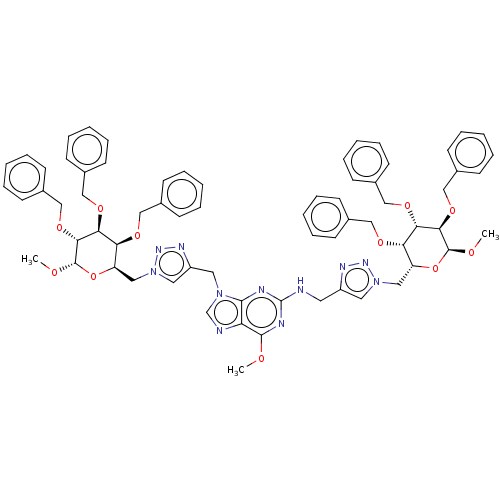
(6-Methoxy-N,9-bis[ (1-{[(2R,3S,4S,5R,6S)-3,4,5-tri...)Show SMILES CO[C@H]1O[C@H](Cn2cc(CNc3nc(OC)c4ncn(Cc5cn(C[C@H]6O[C@H](OC)[C@H](OCc7ccccc7)[C@@H](OCc7ccccc7)[C@H]6OCc6ccccc6)nn5)c4n3)nn2)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C68H73N11O11/c1-80-65-57-64(77(46-70-57)35-54-37-79(76-74-54)39-56-59(84-41-48-24-12-5-13-25-48)61(86-43-50-28-16-7-17-29-50)63(67(82-3)90-56)88-45-52-32-20-9-21-33-52)71-68(72-65)69-34-53-36-78(75-73-53)38-55-58(83-40-47-22-10-4-11-23-47)60(85-42-49-26-14-6-15-27-49)62(66(81-2)89-55)87-44-51-30-18-8-19-31-51/h4-33,36-37,46,55-56,58-63,66-67H,34-35,38-45H2,1-3H3,(H,69,71,72)/t55-,56-,58+,59+,60+,61+,62-,63-,66+,67+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
East China University of Science and Technology
| Assay Description
Enzymatic activity of enzyme was evaluated using pNPP assay. Briefly, a reaction mixture (100 無) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and recom... |
Chem Biodivers 8: 2035-44 (2011)
Article DOI: 10.1002/cbdv.201000242
BindingDB Entry DOI: 10.7270/Q2668BVX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM125935
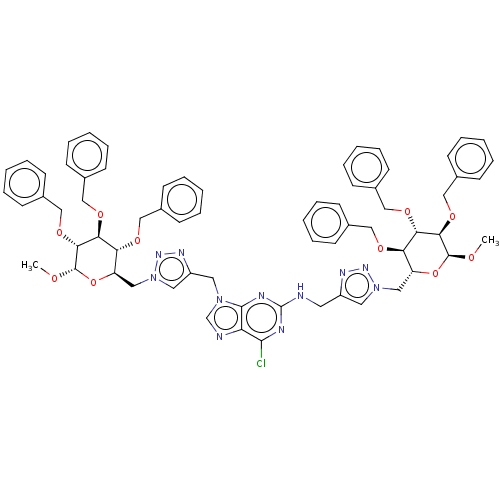
(6-Chloro-N,9-bis[(1-{[(2R,3R,4S,5R,6S)-3,4,5-tris(...)Show SMILES CO[C@H]1O[C@H](Cn2cc(CNc3nc(Cl)c4ncn(Cc5cn(C[C@H]6O[C@H](OC)[C@H](OCc7ccccc7)[C@@H](OCc7ccccc7)[C@@H]6OCc6ccccc6)nn5)c4n3)nn2)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C67H70ClN11O10/c1-80-65-61(86-43-50-29-17-7-18-30-50)59(84-41-48-25-13-5-14-26-48)57(82-39-46-21-9-3-10-22-46)54(88-65)37-78-35-52(73-75-78)33-69-67-71-63(68)56-64(72-67)77(45-70-56)34-53-36-79(76-74-53)38-55-58(83-40-47-23-11-4-12-24-47)60(85-42-49-27-15-6-16-28-49)62(66(81-2)89-55)87-44-51-31-19-8-20-32-51/h3-32,35-36,45,54-55,57-62,65-66H,33-34,37-44H2,1-2H3,(H,69,71,72)/t54-,55-,57-,58-,59+,60+,61-,62-,65+,66+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
East China University of Science and Technology
| Assay Description
Enzymatic activity of enzyme was evaluated using pNPP assay. Briefly, a reaction mixture (100 無) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and recom... |
Chem Biodivers 8: 2035-44 (2011)
Article DOI: 10.1002/cbdv.201000242
BindingDB Entry DOI: 10.7270/Q2668BVX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50245899
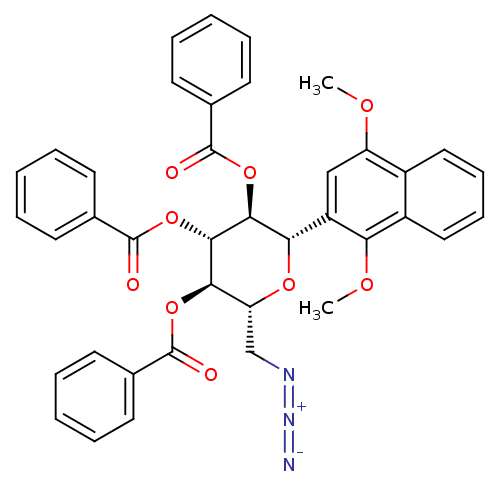
((2R,3R,4R,5S,6S)-2-(azidomethyl)-6-(1,4-dimethoxyn...)Show SMILES COc1cc([C@@H]2O[C@H](CN=[N+]=[N-])[C@@H](OC(=O)c3ccccc3)[C@H](OC(=O)c3ccccc3)[C@H]2OC(=O)c2ccccc2)c(OC)c2ccccc12 |r| Show InChI InChI=1S/C39H33N3O9/c1-46-30-22-29(32(47-2)28-21-13-12-20-27(28)30)33-35(50-38(44)25-16-8-4-9-17-25)36(51-39(45)26-18-10-5-11-19-26)34(31(48-33)23-41-42-40)49-37(43)24-14-6-3-7-15-24/h3-22,31,33-36H,23H2,1-2H3/t31-,33+,34-,35+,36+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PTP1B |
Bioorg Med Chem Lett 18: 6348-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.091
BindingDB Entry DOI: 10.7270/Q28W3F68 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50245898
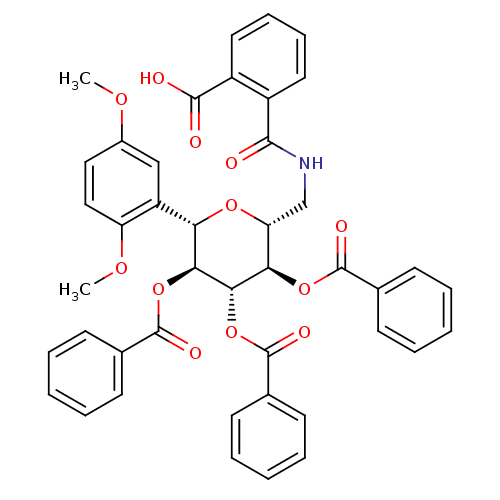
(2-((((2R,3R,4R,5S,6S)-3,4,5-tris(benzoyloxy)-6-(2,...)Show SMILES COc1ccc(OC)c(c1)[C@@H]1O[C@H](CNC(=O)c2ccccc2C(O)=O)[C@@H](OC(=O)c2ccccc2)[C@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1 |r| Show InChI InChI=1S/C43H37NO12/c1-51-29-22-23-33(52-2)32(24-29)35-37(55-42(49)27-16-8-4-9-17-27)38(56-43(50)28-18-10-5-11-19-28)36(54-41(48)26-14-6-3-7-15-26)34(53-35)25-44-39(45)30-20-12-13-21-31(30)40(46)47/h3-24,34-38H,25H2,1-2H3,(H,44,45)(H,46,47)/t34-,35+,36-,37+,38+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PTP1B |
Bioorg Med Chem Lett 18: 6348-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.091
BindingDB Entry DOI: 10.7270/Q28W3F68 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM125938

(6-Methoxy-N,9-bis[ (1-{[(2R,3S,4S,5R,6S)-3,4,5-tri...)Show SMILES CO[C@H]1O[C@H](Cn2cc(CNc3nc(OC)c4ncn(Cc5cn(C[C@H]6O[C@H](OC)[C@H](OCc7ccccc7)[C@@H](OCc7ccccc7)[C@H]6OCc6ccccc6)nn5)c4n3)nn2)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C68H73N11O11/c1-80-65-57-64(77(46-70-57)35-54-37-79(76-74-54)39-56-59(84-41-48-24-12-5-13-25-48)61(86-43-50-28-16-7-17-29-50)63(67(82-3)90-56)88-45-52-32-20-9-21-33-52)71-68(72-65)69-34-53-36-78(75-73-53)38-55-58(83-40-47-22-10-4-11-23-47)60(85-42-49-26-14-6-15-27-49)62(66(81-2)89-55)87-44-51-30-18-8-19-31-51/h4-33,36-37,46,55-56,58-63,66-67H,34-35,38-45H2,1-3H3,(H,69,71,72)/t55-,56-,58+,59+,60+,61+,62-,63-,66+,67+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
East China University of Science and Technology
| Assay Description
Enzymatic activity of enzyme was evaluated using pNPP assay. Briefly, a reaction mixture (100 無) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and recom... |
Chem Biodivers 8: 2035-44 (2011)
Article DOI: 10.1002/cbdv.201000242
BindingDB Entry DOI: 10.7270/Q2668BVX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM125936
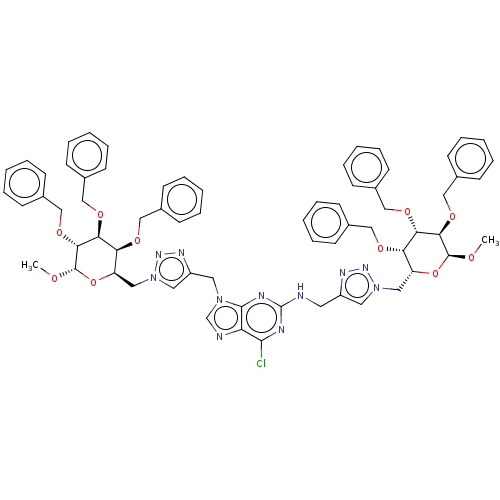
(6-Chloro-N,9-bis[(1-{[(2R,3S,4S,5R,6S)-3,4,5-tris(...)Show SMILES CO[C@H]1O[C@H](Cn2cc(CNc3nc(Cl)c4ncn(Cc5cn(C[C@H]6O[C@H](OC)[C@H](OCc7ccccc7)[C@@H](OCc7ccccc7)[C@H]6OCc6ccccc6)nn5)c4n3)nn2)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C67H70ClN11O10/c1-80-65-61(86-43-50-29-17-7-18-30-50)59(84-41-48-25-13-5-14-26-48)57(82-39-46-21-9-3-10-22-46)54(88-65)37-78-35-52(73-75-78)33-69-67-71-63(68)56-64(72-67)77(45-70-56)34-53-36-79(76-74-53)38-55-58(83-40-47-23-11-4-12-24-47)60(85-42-49-27-15-6-16-28-49)62(66(81-2)89-55)87-44-51-31-19-8-20-32-51/h3-32,35-36,45,54-55,57-62,65-66H,33-34,37-44H2,1-2H3,(H,69,71,72)/t54-,55-,57+,58+,59+,60+,61-,62-,65+,66+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
East China University of Science and Technology
| Assay Description
Enzymatic activity of enzyme was evaluated using pNPP assay. Briefly, a reaction mixture (100 無) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and recom... |
Chem Biodivers 8: 2035-44 (2011)
Article DOI: 10.1002/cbdv.201000242
BindingDB Entry DOI: 10.7270/Q2668BVX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124228
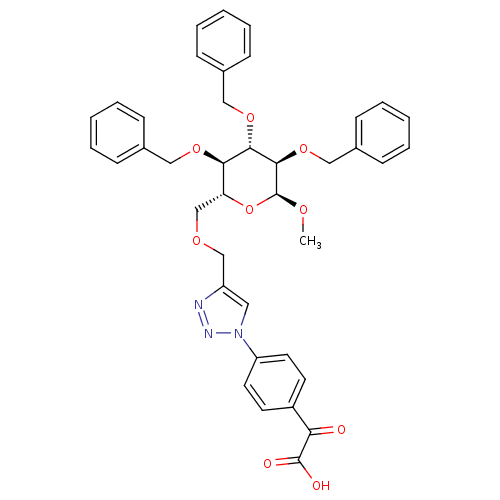
(Methyl 2,3,4-tri-O-benzyl-6-O-[1-(4- (carboxycarbo...)Show SMILES CO[C@H]1O[C@H](COCc2cn(nn2)-c2ccc(cc2)C(=O)C(O)=O)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C39H39N3O9/c1-46-39-37(50-24-29-15-9-4-10-16-29)36(49-23-28-13-7-3-8-14-28)35(48-22-27-11-5-2-6-12-27)33(51-39)26-47-25-31-21-42(41-40-31)32-19-17-30(18-20-32)34(43)38(44)45/h2-21,33,35-37,39H,22-26H2,1H3,(H,44,45)/t33-,35-,36+,37-,39+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | 30 |
East China University of Science and Technology
| Assay Description
The assay mixture (100 ??L) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and PTP1B were monitored at 405 nm for 2 min at 30 ??C. |
Carbohydr Res 346: 140-5 (2011)
Article DOI: 10.1016/j.carres.2010.10.023
BindingDB Entry DOI: 10.7270/Q2T43RRJ |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50336340
(CHEMBL1667958 | Triazole tetra-benzyloxy-beta-D-O-...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)n1cc(CO[C@@H]2O[C@H](COCc3ccccc3)[C@@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 |r| Show InChI InChI=1S/C46H47N3O9/c50-39-23-21-33(22-24-39)25-40(45(51)52)49-26-38(47-48-49)31-57-46-44(56-30-37-19-11-4-12-20-37)43(55-29-36-17-9-3-10-18-36)42(54-28-35-15-7-2-8-16-35)41(58-46)32-53-27-34-13-5-1-6-14-34/h1-24,26,40-44,46,50H,25,27-32H2,(H,51,52)/t40-,41+,42+,43-,44+,46+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CDC25B by pNPP hydrolysis assay |
Bioorg Med Chem Lett 21: 1092-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.126
BindingDB Entry DOI: 10.7270/Q2D79BP8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50245901
((2S,3S,4S,5R,6R)-6-(benzamidomethyl)-2-(2,5-dimeth...)Show SMILES COc1ccc(OC)c(c1)[C@@H]1O[C@H](CNC(=O)c2ccccc2)[C@@H](O)[C@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H33NO9/c1-41-25-18-19-27(42-2)26(20-25)30-32(45-35(40)24-16-10-5-11-17-24)31(44-34(39)23-14-8-4-9-15-23)29(37)28(43-30)21-36-33(38)22-12-6-3-7-13-22/h3-20,28-32,37H,21H2,1-2H3,(H,36,38)/t28-,29-,30+,31+,32+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PTP1B |
Bioorg Med Chem Lett 18: 6348-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.091
BindingDB Entry DOI: 10.7270/Q28W3F68 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50246114
((2S,3S,4R,5R,6R)-5-acetoxy-6-(benzamidomethyl)-2-(...)Show SMILES COc1ccc(OC)c(c1)[C@@H]1O[C@H](CNC(=O)c2ccccc2)[C@@H](OC(C)=O)[C@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1 |r| Show InChI InChI=1S/C37H35NO10/c1-23(39)45-32-30(22-38-35(40)24-13-7-4-8-14-24)46-31(28-21-27(43-2)19-20-29(28)44-3)33(47-36(41)25-15-9-5-10-16-25)34(32)48-37(42)26-17-11-6-12-18-26/h4-21,30-34H,22H2,1-3H3,(H,38,40)/t30-,31+,32-,33+,34+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PTP1B |
Bioorg Med Chem Lett 18: 6348-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.091
BindingDB Entry DOI: 10.7270/Q28W3F68 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50273791
(Acetic acid (2R,3R,4R,5S,6S)-3,4,5-triacetoxy-6-(3...)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)C1=CC(=O)C=CC1=O |r,c:28,t:24| Show InChI InChI=1S/C20H22O11/c1-9(21)27-8-16-18(28-10(2)22)20(30-12(4)24)19(29-11(3)23)17(31-16)14-7-13(25)5-6-15(14)26/h5-7,16-20H,8H2,1-4H3/t16-,17+,18-,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PTP1B |
Bioorg Med Chem Lett 18: 6348-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.091
BindingDB Entry DOI: 10.7270/Q28W3F68 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50273791

(Acetic acid (2R,3R,4R,5S,6S)-3,4,5-triacetoxy-6-(3...)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)C1=CC(=O)C=CC1=O |r,c:28,t:24| Show InChI InChI=1S/C20H22O11/c1-9(21)27-8-16-18(28-10(2)22)20(30-12(4)24)19(29-11(3)23)17(31-16)14-7-13(25)5-6-15(14)26/h5-7,16-20H,8H2,1-4H3/t16-,17+,18-,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B catalytic domain |
Bioorg Med Chem 16: 9757-63 (2008)
Article DOI: 10.1016/j.bmc.2008.09.066
BindingDB Entry DOI: 10.7270/Q2G73DJN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50336339
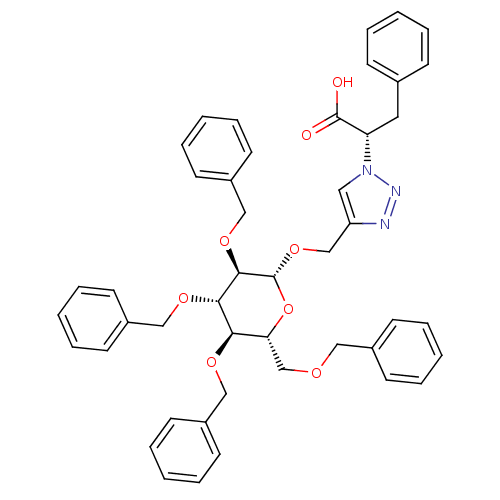
((S)-3-phenyl-2-(4-(((2R,3R,4S,5R,6R)-3,4,5-tris(be...)Show SMILES OC(=O)[C@H](Cc1ccccc1)n1cc(CO[C@@H]2O[C@H](COCc3ccccc3)[C@@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 |r| Show InChI InChI=1S/C46H47N3O8/c50-45(51)40(26-34-16-6-1-7-17-34)49-27-39(47-48-49)32-56-46-44(55-31-38-24-14-5-15-25-38)43(54-30-37-22-12-4-13-23-37)42(53-29-36-20-10-3-11-21-36)41(57-46)33-52-28-35-18-8-2-9-19-35/h1-25,27,40-44,46H,26,28-33H2,(H,50,51)/t40-,41+,42+,43-,44+,46+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B by pNPP hydrolysis assay |
Bioorg Med Chem Lett 21: 1092-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.126
BindingDB Entry DOI: 10.7270/Q2D79BP8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50246115
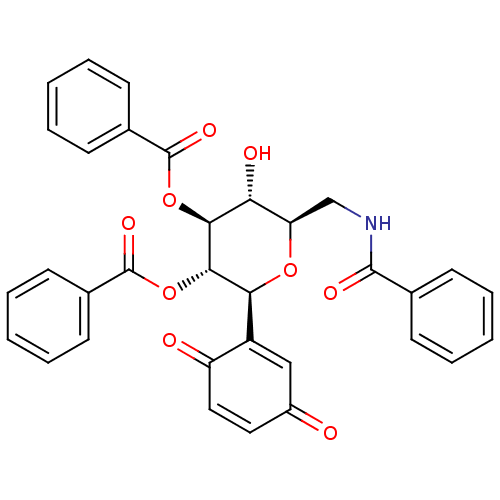
((2S,3S,4S,5R,6R)-6-(benzamidomethyl)-2-(3,6-dioxoc...)Show SMILES O[C@@H]1[C@@H](CNC(=O)c2ccccc2)O[C@H]([C@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1)C1=CC(=O)C=CC1=O |r,c:43,t:39| Show InChI InChI=1S/C33H27NO9/c35-23-16-17-25(36)24(18-23)28-30(43-33(40)22-14-8-3-9-15-22)29(42-32(39)21-12-6-2-7-13-21)27(37)26(41-28)19-34-31(38)20-10-4-1-5-11-20/h1-18,26-30,37H,19H2,(H,34,38)/t26-,27-,28+,29+,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PTP1B |
Bioorg Med Chem Lett 18: 6348-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.091
BindingDB Entry DOI: 10.7270/Q28W3F68 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124232
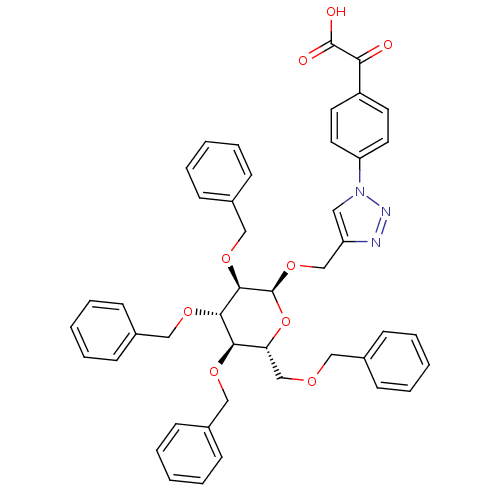
(1-(4-(Carboxycarbonyl)phenyl)-1H-1,2,3-triazol-4- ...)Show SMILES OC(=O)C(=O)c1ccc(cc1)-n1cc(CO[C@H]2O[C@H](COCc3ccccc3)[C@@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 Show InChI InChI=1S/C45H43N3O9/c49-40(44(50)51)36-21-23-38(24-22-36)48-25-37(46-47-48)30-56-45-43(55-29-35-19-11-4-12-20-35)42(54-28-34-17-9-3-10-18-34)41(53-27-33-15-7-2-8-16-33)39(57-45)31-52-26-32-13-5-1-6-14-32/h1-25,39,41-43,45H,26-31H2,(H,50,51)/t39-,41-,42+,43-,45+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | 30 |
East China University of Science and Technology
| Assay Description
The assay mixture (100 ??L) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and PTP1B were monitored at 405 nm for 2 min at 30 ??C. |
Carbohydr Res 346: 140-5 (2011)
Article DOI: 10.1016/j.carres.2010.10.023
BindingDB Entry DOI: 10.7270/Q2T43RRJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50347243

(CHEMBL1795950)Show SMILES CC(C)(C)OC(=O)N[C@@H](COCc1cn(nn1)[C@@H]1O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C45H52N4O10/c1-45(2,3)59-44(52)46-37(43(50)51)30-54-29-36-24-49(48-47-36)42-41(57-28-35-22-14-7-15-23-35)40(56-27-34-20-12-6-13-21-34)39(55-26-33-18-10-5-11-19-33)38(58-42)31-53-25-32-16-8-4-9-17-32/h4-24,37-42H,25-31H2,1-3H3,(H,46,52)(H,50,51)/t37-,38+,39+,40-,41+,42+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTB1B catalytic domain using pNPP substrate |
Bioorg Med Chem 19: 3892-900 (2011)
Article DOI: 10.1016/j.bmc.2011.05.049
BindingDB Entry DOI: 10.7270/Q2RJ4JTV |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50347243

(CHEMBL1795950)Show SMILES CC(C)(C)OC(=O)N[C@@H](COCc1cn(nn1)[C@@H]1O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C45H52N4O10/c1-45(2,3)59-44(52)46-37(43(50)51)30-54-29-36-24-49(48-47-36)42-41(57-28-35-22-14-7-15-23-35)40(56-27-34-20-12-6-13-21-34)39(55-26-33-18-10-5-11-19-33)38(58-42)31-53-25-32-16-8-4-9-17-32/h4-24,37-42H,25-31H2,1-3H3,(H,46,52)(H,50,51)/t37-,38+,39+,40-,41+,42+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25B |
Bioorg Med Chem 19: 3892-900 (2011)
Article DOI: 10.1016/j.bmc.2011.05.049
BindingDB Entry DOI: 10.7270/Q2RJ4JTV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM124232

(1-(4-(Carboxycarbonyl)phenyl)-1H-1,2,3-triazol-4- ...)Show SMILES OC(=O)C(=O)c1ccc(cc1)-n1cc(CO[C@H]2O[C@H](COCc3ccccc3)[C@@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 Show InChI InChI=1S/C45H43N3O9/c49-40(44(50)51)36-21-23-38(24-22-36)48-25-37(46-47-48)30-56-45-43(55-29-35-19-11-4-12-20-35)42(54-28-34-17-9-3-10-18-34)41(53-27-33-15-7-2-8-16-33)39(57-45)31-52-26-32-13-5-1-6-14-32/h1-25,39,41-43,45H,26-31H2,(H,50,51)/t39-,41-,42+,43-,45+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | 30 |
East China University of Science and Technology
| Assay Description
The assay mixture (100 ??L) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and PTP1B were monitored at 405 nm for 2 min at 30 ??C. |
Carbohydr Res 346: 140-5 (2011)
Article DOI: 10.1016/j.carres.2010.10.023
BindingDB Entry DOI: 10.7270/Q2T43RRJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50351999
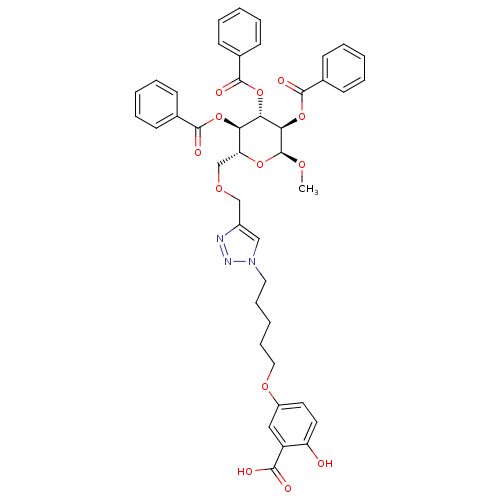
(CHEMBL1822607)Show SMILES CO[C@H]1O[C@H](COCc2cn(CCCCCOc3ccc(O)c(c3)C(O)=O)nn2)[C@@H](OC(=O)c2ccccc2)[C@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1 |r| Show InChI InChI=1S/C43H43N3O13/c1-53-43-38(59-42(52)30-18-10-4-11-19-30)37(58-41(51)29-16-8-3-9-17-29)36(57-40(50)28-14-6-2-7-15-28)35(56-43)27-54-26-31-25-46(45-44-31)22-12-5-13-23-55-32-20-21-34(47)33(24-32)39(48)49/h2-4,6-11,14-21,24-25,35-38,43,47H,5,12-13,22-23,26-27H2,1H3,(H,48,49)/t35-,36-,37+,38-,43+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in CHO cells assessed as p-nitorphenol production after 2 mins by microplate reader |
Eur J Med Chem 46: 4212-8 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.025
BindingDB Entry DOI: 10.7270/Q2FT8MDS |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50336341
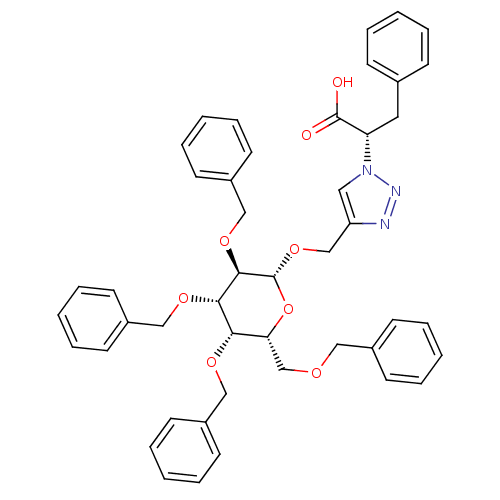
(CHEMBL1667959 | Triazole tetra-benzyloxy-beta-D-O-...)Show SMILES OC(=O)[C@H](Cc1ccccc1)n1cc(CO[C@@H]2O[C@H](COCc3ccccc3)[C@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 |r| Show InChI InChI=1S/C46H47N3O8/c50-45(51)40(26-34-16-6-1-7-17-34)49-27-39(47-48-49)32-56-46-44(55-31-38-24-14-5-15-25-38)43(54-30-37-22-12-4-13-23-37)42(53-29-36-20-10-3-11-21-36)41(57-46)33-52-28-35-18-8-2-9-19-35/h1-25,27,40-44,46H,26,28-33H2,(H,50,51)/t40-,41+,42-,43-,44+,46+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CDC25B by pNPP hydrolysis assay |
Bioorg Med Chem Lett 21: 1092-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.126
BindingDB Entry DOI: 10.7270/Q2D79BP8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50336341

(CHEMBL1667959 | Triazole tetra-benzyloxy-beta-D-O-...)Show SMILES OC(=O)[C@H](Cc1ccccc1)n1cc(CO[C@@H]2O[C@H](COCc3ccccc3)[C@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 |r| Show InChI InChI=1S/C46H47N3O8/c50-45(51)40(26-34-16-6-1-7-17-34)49-27-39(47-48-49)32-56-46-44(55-31-38-24-14-5-15-25-38)43(54-30-37-22-12-4-13-23-37)42(53-29-36-20-10-3-11-21-36)41(57-46)33-52-28-35-18-8-2-9-19-35/h1-25,27,40-44,46H,26,28-33H2,(H,50,51)/t40-,41+,42-,43-,44+,46+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B by pNPP hydrolysis assay |
Bioorg Med Chem Lett 21: 1092-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.126
BindingDB Entry DOI: 10.7270/Q2D79BP8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50347244
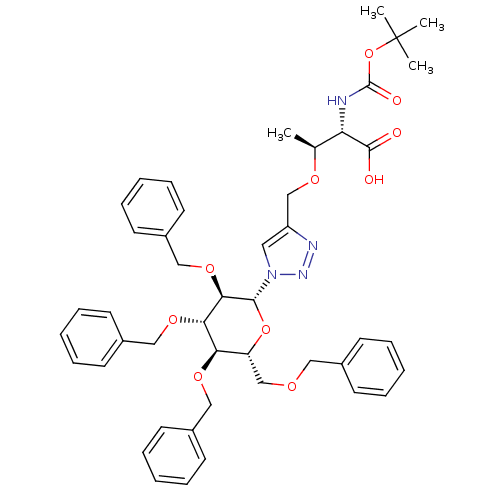
(CHEMBL1795951)Show SMILES C[C@H](OCc1cn(nn1)[C@@H]1O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1)[C@H](NC(=O)OC(C)(C)C)C(O)=O |r| Show InChI InChI=1S/C46H54N4O10/c1-32(39(44(51)52)47-45(53)60-46(2,3)4)55-30-37-25-50(49-48-37)43-42(58-29-36-23-15-8-16-24-36)41(57-28-35-21-13-7-14-22-35)40(56-27-34-19-11-6-12-20-34)38(59-43)31-54-26-33-17-9-5-10-18-33/h5-25,32,38-43H,26-31H2,1-4H3,(H,47,53)(H,51,52)/t32-,38+,39-,40+,41-,42+,43+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTB1B catalytic domain using pNPP substrate |
Bioorg Med Chem 19: 3892-900 (2011)
Article DOI: 10.1016/j.bmc.2011.05.049
BindingDB Entry DOI: 10.7270/Q2RJ4JTV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
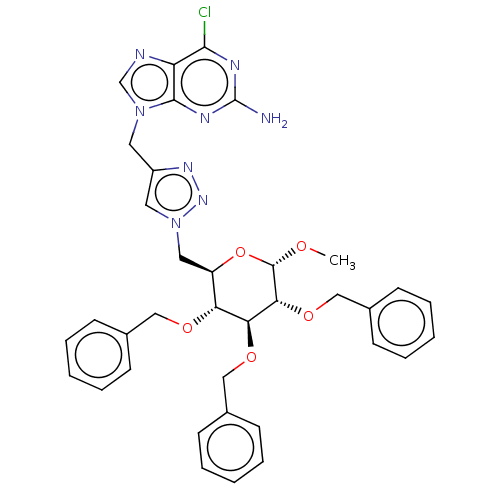
(Homo sapiens (Human)) | BDBM125931
(Methyl 6-{4-[(2-Amino-6-chloro-9H-purin-9-yl)methy...)Show SMILES CO[C@H]1O[C@H](Cn2cc(Cn3cnc4c(Cl)nc(N)nc34)nn2)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C36H37ClN8O5/c1-46-35-32(49-22-26-15-9-4-10-16-26)31(48-21-25-13-7-3-8-14-25)30(47-20-24-11-5-2-6-12-24)28(50-35)19-45-18-27(42-43-45)17-44-23-39-29-33(37)40-36(38)41-34(29)44/h2-16,18,23,28,30-32,35H,17,19-22H2,1H3,(H2,38,40,41)/t28-,30-,31+,32-,35+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
East China University of Science and Technology
| Assay Description
Enzymatic activity of enzyme was evaluated using pNPP assay. Briefly, a reaction mixture (100 無) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and recom... |
Chem Biodivers 8: 2035-44 (2011)
Article DOI: 10.1002/cbdv.201000242
BindingDB Entry DOI: 10.7270/Q2668BVX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50336339

((S)-3-phenyl-2-(4-(((2R,3R,4S,5R,6R)-3,4,5-tris(be...)Show SMILES OC(=O)[C@H](Cc1ccccc1)n1cc(CO[C@@H]2O[C@H](COCc3ccccc3)[C@@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 |r| Show InChI InChI=1S/C46H47N3O8/c50-45(51)40(26-34-16-6-1-7-17-34)49-27-39(47-48-49)32-56-46-44(55-31-38-24-14-5-15-25-38)43(54-30-37-22-12-4-13-23-37)42(53-29-36-20-10-3-11-21-36)41(57-46)33-52-28-35-18-8-2-9-19-35/h1-25,27,40-44,46H,26,28-33H2,(H,50,51)/t40-,41+,42+,43-,44+,46+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human TCPTP by pNPP hydrolysis assay |
Bioorg Med Chem Lett 21: 1092-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.126
BindingDB Entry DOI: 10.7270/Q2D79BP8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM125937
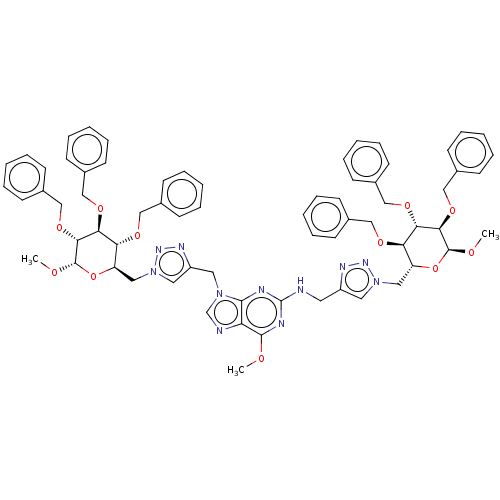
(6-Methoxy-N,9-bis[(1-{[(2R,3R,4S,5R,6S)-3,4,5-tris...)Show SMILES CO[C@H]1O[C@H](Cn2cc(CNc3nc(OC)c4ncn(Cc5cn(C[C@H]6O[C@H](OC)[C@H](OCc7ccccc7)[C@@H](OCc7ccccc7)[C@@H]6OCc6ccccc6)nn5)c4n3)nn2)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C68H73N11O11/c1-80-65-57-64(77(46-70-57)35-54-37-79(76-74-54)39-56-59(84-41-48-24-12-5-13-25-48)61(86-43-50-28-16-7-17-29-50)63(67(82-3)90-56)88-45-52-32-20-9-21-33-52)71-68(72-65)69-34-53-36-78(75-73-53)38-55-58(83-40-47-22-10-4-11-23-47)60(85-42-49-26-14-6-15-27-49)62(66(81-2)89-55)87-44-51-30-18-8-19-31-51/h4-33,36-37,46,55-56,58-63,66-67H,34-35,38-45H2,1-3H3,(H,69,71,72)/t55-,56-,58-,59-,60+,61+,62-,63-,66+,67+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
East China University of Science and Technology
| Assay Description
Enzymatic activity of enzyme was evaluated using pNPP assay. Briefly, a reaction mixture (100 無) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and recom... |
Chem Biodivers 8: 2035-44 (2011)
Article DOI: 10.1002/cbdv.201000242
BindingDB Entry DOI: 10.7270/Q2668BVX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50347247
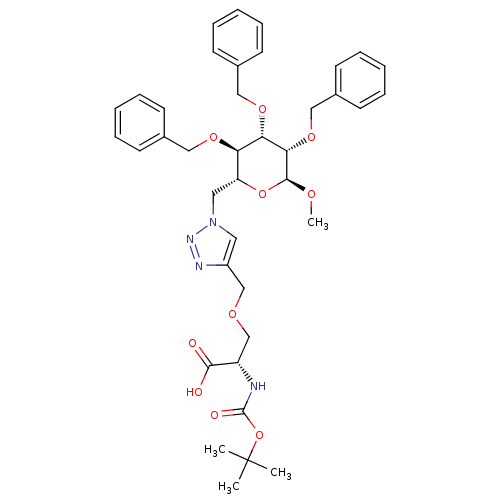
(CHEMBL1795948)Show SMILES CO[C@H]1O[C@H](Cn2cc(COC[C@H](NC(=O)OC(C)(C)C)C(O)=O)nn2)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C39H48N4O10/c1-39(2,3)53-38(46)40-31(36(44)45)26-48-25-30-20-43(42-41-30)21-32-33(49-22-27-14-8-5-9-15-27)34(50-23-28-16-10-6-11-17-28)35(37(47-4)52-32)51-24-29-18-12-7-13-19-29/h5-20,31-35,37H,21-26H2,1-4H3,(H,40,46)(H,44,45)/t31-,32+,33+,34-,35-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25B |
Bioorg Med Chem 19: 3892-900 (2011)
Article DOI: 10.1016/j.bmc.2011.05.049
BindingDB Entry DOI: 10.7270/Q2RJ4JTV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50336340

(CHEMBL1667958 | Triazole tetra-benzyloxy-beta-D-O-...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)n1cc(CO[C@@H]2O[C@H](COCc3ccccc3)[C@@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 |r| Show InChI InChI=1S/C46H47N3O9/c50-39-23-21-33(22-24-39)25-40(45(51)52)49-26-38(47-48-49)31-57-46-44(56-30-37-19-11-4-12-20-37)43(55-29-36-17-9-3-10-18-36)42(54-28-35-15-7-2-8-16-35)41(58-46)32-53-27-34-13-5-1-6-14-34/h1-24,26,40-44,46,50H,25,27-32H2,(H,51,52)/t40-,41+,42+,43-,44+,46+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B by pNPP hydrolysis assay |
Bioorg Med Chem Lett 21: 1092-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.126
BindingDB Entry DOI: 10.7270/Q2D79BP8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50351998
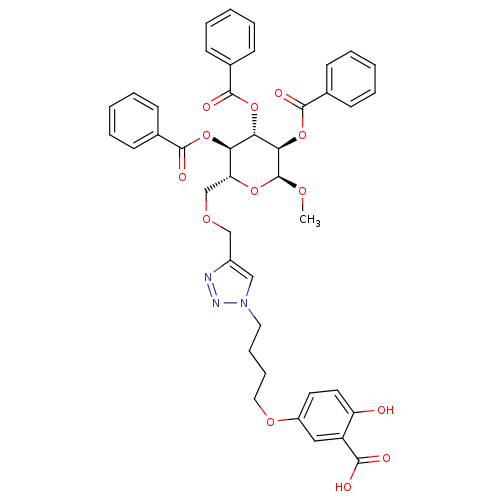
(CHEMBL1822606)Show SMILES CO[C@H]1O[C@H](COCc2cn(CCCCOc3ccc(O)c(c3)C(O)=O)nn2)[C@@H](OC(=O)c2ccccc2)[C@H](OC(=O)c2ccccc2)[C@H]1OC(=O)c1ccccc1 |r| Show InChI InChI=1S/C42H41N3O13/c1-52-42-37(58-41(51)29-17-9-4-10-18-29)36(57-40(50)28-15-7-3-8-16-28)35(56-39(49)27-13-5-2-6-14-27)34(55-42)26-53-25-30-24-45(44-43-30)21-11-12-22-54-31-19-20-33(46)32(23-31)38(47)48/h2-10,13-20,23-24,34-37,42,46H,11-12,21-22,25-26H2,1H3,(H,47,48)/t34-,35-,36+,37-,42+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in CHO cells assessed as p-nitorphenol production after 2 mins by microplate reader |
Eur J Med Chem 46: 4212-8 (2011)
Article DOI: 10.1016/j.ejmech.2011.06.025
BindingDB Entry DOI: 10.7270/Q2FT8MDS |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50336339

((S)-3-phenyl-2-(4-(((2R,3R,4S,5R,6R)-3,4,5-tris(be...)Show SMILES OC(=O)[C@H](Cc1ccccc1)n1cc(CO[C@@H]2O[C@H](COCc3ccccc3)[C@@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 |r| Show InChI InChI=1S/C46H47N3O8/c50-45(51)40(26-34-16-6-1-7-17-34)49-27-39(47-48-49)32-56-46-44(55-31-38-24-14-5-15-25-38)43(54-30-37-22-12-4-13-23-37)42(53-29-36-20-10-3-11-21-36)41(57-46)33-52-28-35-18-8-2-9-19-35/h1-25,27,40-44,46H,26,28-33H2,(H,50,51)/t40-,41+,42+,43-,44+,46+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CDC25B by pNPP hydrolysis assay |
Bioorg Med Chem Lett 21: 1092-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.126
BindingDB Entry DOI: 10.7270/Q2D79BP8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM125935

(6-Chloro-N,9-bis[(1-{[(2R,3R,4S,5R,6S)-3,4,5-tris(...)Show SMILES CO[C@H]1O[C@H](Cn2cc(CNc3nc(Cl)c4ncn(Cc5cn(C[C@H]6O[C@H](OC)[C@H](OCc7ccccc7)[C@@H](OCc7ccccc7)[C@@H]6OCc6ccccc6)nn5)c4n3)nn2)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C67H70ClN11O10/c1-80-65-61(86-43-50-29-17-7-18-30-50)59(84-41-48-25-13-5-14-26-48)57(82-39-46-21-9-3-10-22-46)54(88-65)37-78-35-52(73-75-78)33-69-67-71-63(68)56-64(72-67)77(45-70-56)34-53-36-79(76-74-53)38-55-58(83-40-47-23-11-4-12-24-47)60(85-42-49-27-15-6-16-28-49)62(66(81-2)89-55)87-44-51-31-19-8-20-32-51/h3-32,35-36,45,54-55,57-62,65-66H,33-34,37-44H2,1-2H3,(H,69,71,72)/t54-,55-,57-,58-,59+,60+,61-,62-,65+,66+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 6.5 | n/a |
East China University of Science and Technology
| Assay Description
Enzymatic activity of enzyme was evaluated using pNPP assay. Briefly, a reaction mixture (100 無) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and recom... |
Chem Biodivers 8: 2035-44 (2011)
Article DOI: 10.1002/cbdv.201000242
BindingDB Entry DOI: 10.7270/Q2668BVX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM125932
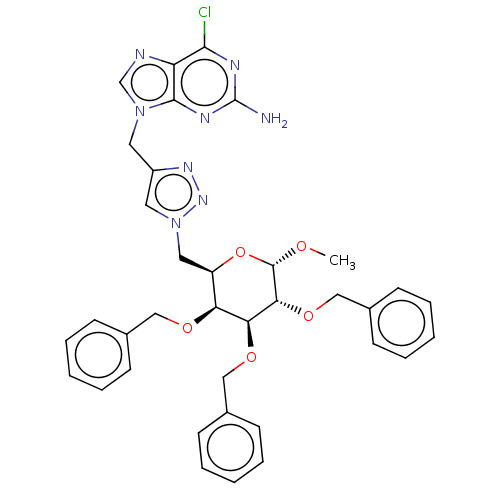
(Methyl 6-{4-[(2-Amino-6-chloro-9H-purin-9-yl)methy...)Show SMILES CO[C@H]1O[C@H](Cn2cc(Cn3cnc4c(Cl)nc(N)nc34)nn2)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C36H37ClN8O5/c1-46-35-32(49-22-26-15-9-4-10-16-26)31(48-21-25-13-7-3-8-14-25)30(47-20-24-11-5-2-6-12-24)28(50-35)19-45-18-27(42-43-45)17-44-23-39-29-33(37)40-36(38)41-34(29)44/h2-16,18,23,28,30-32,35H,17,19-22H2,1H3,(H2,38,40,41)/t28-,30+,31+,32-,35+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | 6.5 | n/a |
East China University of Science and Technology
| Assay Description
Enzymatic activity of enzyme was evaluated using pNPP assay. Briefly, a reaction mixture (100 無) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and recom... |
Chem Biodivers 8: 2035-44 (2011)
Article DOI: 10.1002/cbdv.201000242
BindingDB Entry DOI: 10.7270/Q2668BVX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124231

(1-(4-Carboxyphenyl)-1H-1,2,3-triazol-4-ylmethyl 2,...)Show SMILES OC(=O)c1ccc(cc1)-n1cc(CO[C@H]2O[C@H](COCc3ccccc3)[C@@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 Show InChI InChI=1S/C44H43N3O8/c48-43(49)36-21-23-38(24-22-36)47-25-37(45-46-47)30-54-44-42(53-29-35-19-11-4-12-20-35)41(52-28-34-17-9-3-10-18-34)40(51-27-33-15-7-2-8-16-33)39(55-44)31-50-26-32-13-5-1-6-14-32/h1-25,39-42,44H,26-31H2,(H,48,49)/t39-,40-,41+,42-,44+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
East China University of Science and Technology
| Assay Description
The assay mixture (100 ??L) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and PTP1B were monitored at 405 nm for 2 min at 30 ??C. |
Carbohydr Res 346: 140-5 (2011)
Article DOI: 10.1016/j.carres.2010.10.023
BindingDB Entry DOI: 10.7270/Q2T43RRJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50336332
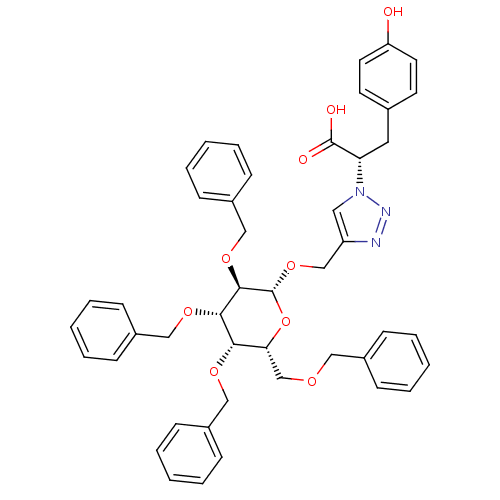
(CHEMBL1667960 | Triazole tetra-benzyloxy-beta-D-O-...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)n1cc(CO[C@@H]2O[C@H](COCc3ccccc3)[C@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 |r| Show InChI InChI=1S/C46H47N3O9/c50-39-23-21-33(22-24-39)25-40(45(51)52)49-26-38(47-48-49)31-57-46-44(56-30-37-19-11-4-12-20-37)43(55-29-36-17-9-3-10-18-36)42(54-28-35-15-7-2-8-16-35)41(58-46)32-53-27-34-13-5-1-6-14-34/h1-24,26,40-44,46,50H,25,27-32H2,(H,51,52)/t40-,41+,42-,43-,44+,46+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B by pNPP hydrolysis assay |
Bioorg Med Chem Lett 21: 1092-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.126
BindingDB Entry DOI: 10.7270/Q2D79BP8 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50347244

(CHEMBL1795951)Show SMILES C[C@H](OCc1cn(nn1)[C@@H]1O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1)[C@H](NC(=O)OC(C)(C)C)C(O)=O |r| Show InChI InChI=1S/C46H54N4O10/c1-32(39(44(51)52)47-45(53)60-46(2,3)4)55-30-37-25-50(49-48-37)43-42(58-29-36-23-15-8-16-24-36)41(57-28-35-21-13-7-14-22-35)40(56-27-34-19-11-6-12-20-34)38(59-43)31-54-26-33-17-9-5-10-18-33/h5-25,32,38-43H,26-31H2,1-4H3,(H,47,53)(H,51,52)/t32-,38+,39-,40+,41-,42+,43+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25B |
Bioorg Med Chem 19: 3892-900 (2011)
Article DOI: 10.1016/j.bmc.2011.05.049
BindingDB Entry DOI: 10.7270/Q2RJ4JTV |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50336332

(CHEMBL1667960 | Triazole tetra-benzyloxy-beta-D-O-...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)n1cc(CO[C@@H]2O[C@H](COCc3ccccc3)[C@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 |r| Show InChI InChI=1S/C46H47N3O9/c50-39-23-21-33(22-24-39)25-40(45(51)52)49-26-38(47-48-49)31-57-46-44(56-30-37-19-11-4-12-20-37)43(55-29-36-17-9-3-10-18-36)42(54-28-35-15-7-2-8-16-35)41(58-46)32-53-27-34-13-5-1-6-14-34/h1-24,26,40-44,46,50H,25,27-32H2,(H,51,52)/t40-,41+,42-,43-,44+,46+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human CDC25B by pNPP hydrolysis assay |
Bioorg Med Chem Lett 21: 1092-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.126
BindingDB Entry DOI: 10.7270/Q2D79BP8 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50347242
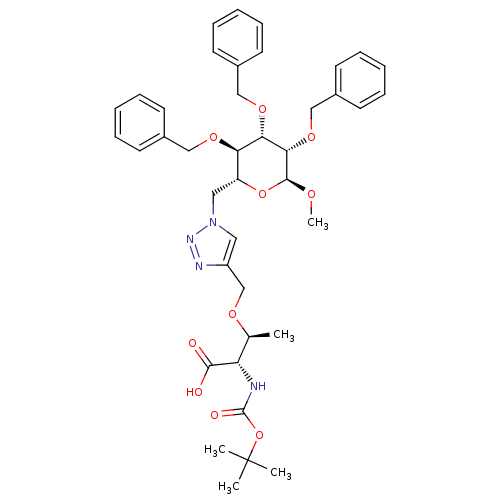
(CHEMBL1795949)Show SMILES CO[C@H]1O[C@H](Cn2cc(CO[C@@H](C)[C@H](NC(=O)OC(C)(C)C)C(O)=O)nn2)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C40H50N4O10/c1-27(33(37(45)46)41-39(47)54-40(2,3)4)49-26-31-21-44(43-42-31)22-32-34(50-23-28-15-9-6-10-16-28)35(51-24-29-17-11-7-12-18-29)36(38(48-5)53-32)52-25-30-19-13-8-14-20-30/h6-21,27,32-36,38H,22-26H2,1-5H3,(H,41,47)(H,45,46)/t27-,32+,33-,34+,35-,36-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human Cdc25B |
Bioorg Med Chem 19: 3892-900 (2011)
Article DOI: 10.1016/j.bmc.2011.05.049
BindingDB Entry DOI: 10.7270/Q2RJ4JTV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124230
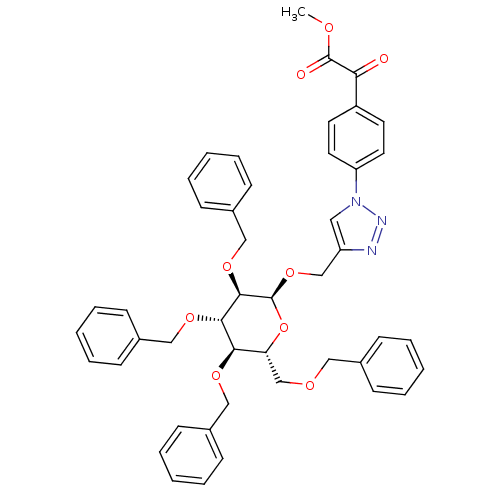
(1-(4-(2-Methoxy-2-oxoacetyl)phenyl)-1H-1,2,3-triaz...)Show SMILES COC(=O)C(=O)c1ccc(cc1)-n1cc(CO[C@H]2O[C@H](COCc3ccccc3)[C@@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 Show InChI InChI=1S/C46H45N3O9/c1-52-45(51)41(50)37-22-24-39(25-23-37)49-26-38(47-48-49)31-57-46-44(56-30-36-20-12-5-13-21-36)43(55-29-35-18-10-4-11-19-35)42(54-28-34-16-8-3-9-17-34)40(58-46)32-53-27-33-14-6-2-7-15-33/h2-26,40,42-44,46H,27-32H2,1H3/t40-,42-,43+,44-,46+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
East China University of Science and Technology
| Assay Description
The assay mixture (100 ??L) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and PTP1B were monitored at 405 nm for 2 min at 30 ??C. |
Carbohydr Res 346: 140-5 (2011)
Article DOI: 10.1016/j.carres.2010.10.023
BindingDB Entry DOI: 10.7270/Q2T43RRJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50347243

(CHEMBL1795950)Show SMILES CC(C)(C)OC(=O)N[C@@H](COCc1cn(nn1)[C@@H]1O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C45H52N4O10/c1-45(2,3)59-44(52)46-37(43(50)51)30-54-29-36-24-49(48-47-36)42-41(57-28-35-22-14-7-15-23-35)40(56-27-34-20-12-6-13-21-34)39(55-26-33-18-10-5-11-19-33)38(58-42)31-53-25-32-16-8-4-9-17-32/h4-24,37-42H,25-31H2,1-3H3,(H,46,52)(H,50,51)/t37-,38+,39+,40-,41+,42+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Bioorg Med Chem 19: 3892-900 (2011)
Article DOI: 10.1016/j.bmc.2011.05.049
BindingDB Entry DOI: 10.7270/Q2RJ4JTV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50336341

(CHEMBL1667959 | Triazole tetra-benzyloxy-beta-D-O-...)Show SMILES OC(=O)[C@H](Cc1ccccc1)n1cc(CO[C@@H]2O[C@H](COCc3ccccc3)[C@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 |r| Show InChI InChI=1S/C46H47N3O8/c50-45(51)40(26-34-16-6-1-7-17-34)49-27-39(47-48-49)32-56-46-44(55-31-38-24-14-5-15-25-38)43(54-30-37-22-12-4-13-23-37)42(53-29-36-20-10-3-11-21-36)41(57-46)33-52-28-35-18-8-2-9-19-35/h1-25,27,40-44,46H,26,28-33H2,(H,50,51)/t40-,41+,42-,43-,44+,46+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human TCPTP by pNPP hydrolysis assay |
Bioorg Med Chem Lett 21: 1092-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.126
BindingDB Entry DOI: 10.7270/Q2D79BP8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124226
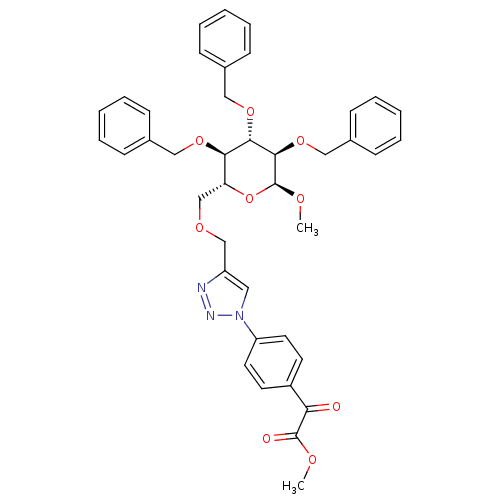
(Methyl 2,3,4-tri-O-benzyl-6-O-[1-(4-(2-methoxy-2- ...)Show SMILES CO[C@H]1O[C@H](COCc2cn(nn2)-c2ccc(cc2)C(=O)C(=O)OC)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C40H41N3O9/c1-46-39(45)35(44)31-18-20-33(21-19-31)43-22-32(41-42-43)26-48-27-34-36(49-23-28-12-6-3-7-13-28)37(50-24-29-14-8-4-9-15-29)38(40(47-2)52-34)51-25-30-16-10-5-11-17-30/h3-22,34,36-38,40H,23-27H2,1-2H3/t34-,36-,37+,38-,40+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
East China University of Science and Technology
| Assay Description
The assay mixture (100 ??L) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and PTP1B were monitored at 405 nm for 2 min at 30 ??C. |
Carbohydr Res 346: 140-5 (2011)
Article DOI: 10.1016/j.carres.2010.10.023
BindingDB Entry DOI: 10.7270/Q2T43RRJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM124228

(Methyl 2,3,4-tri-O-benzyl-6-O-[1-(4- (carboxycarbo...)Show SMILES CO[C@H]1O[C@H](COCc2cn(nn2)-c2ccc(cc2)C(=O)C(O)=O)[C@@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 Show InChI InChI=1S/C39H39N3O9/c1-46-39-37(50-24-29-15-9-4-10-16-29)36(49-23-28-13-7-3-8-14-28)35(48-22-27-11-5-2-6-12-27)33(51-39)26-47-25-31-21-42(41-40-31)32-19-17-30(18-20-32)34(43)38(44)45/h2-21,33,35-37,39H,22-26H2,1H3,(H,44,45)/t33-,35-,36+,37-,39+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | 30 |
East China University of Science and Technology
| Assay Description
The assay mixture (100 ??L) containing 50 mM MOPS (pH 6.5), 2 mM pNPP and PTP1B were monitored at 405 nm for 2 min at 30 ??C. |
Carbohydr Res 346: 140-5 (2011)
Article DOI: 10.1016/j.carres.2010.10.023
BindingDB Entry DOI: 10.7270/Q2T43RRJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50347246
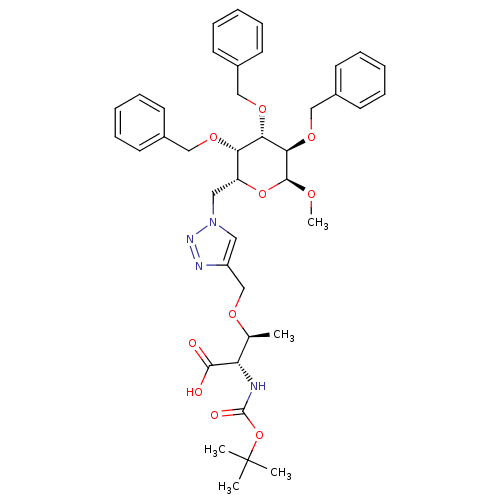
(CHEMBL1795947)Show SMILES CO[C@H]1O[C@H](Cn2cc(CO[C@@H](C)[C@H](NC(=O)OC(C)(C)C)C(O)=O)nn2)[C@H](OCc2ccccc2)[C@H](OCc2ccccc2)[C@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C40H50N4O10/c1-27(33(37(45)46)41-39(47)54-40(2,3)4)49-26-31-21-44(43-42-31)22-32-34(50-23-28-15-9-6-10-16-28)35(51-24-29-17-11-7-12-18-29)36(38(48-5)53-32)52-25-30-19-13-8-14-20-30/h6-21,27,32-36,38H,22-26H2,1-5H3,(H,41,47)(H,45,46)/t27-,32+,33-,34-,35-,36+,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTB1B catalytic domain using pNPP substrate |
Bioorg Med Chem 19: 3892-900 (2011)
Article DOI: 10.1016/j.bmc.2011.05.049
BindingDB Entry DOI: 10.7270/Q2RJ4JTV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50336339

((S)-3-phenyl-2-(4-(((2R,3R,4S,5R,6R)-3,4,5-tris(be...)Show SMILES OC(=O)[C@H](Cc1ccccc1)n1cc(CO[C@@H]2O[C@H](COCc3ccccc3)[C@@H](OCc3ccccc3)[C@H](OCc3ccccc3)[C@H]2OCc2ccccc2)nn1 |r| Show InChI InChI=1S/C46H47N3O8/c50-45(51)40(26-34-16-6-1-7-17-34)49-27-39(47-48-49)32-56-46-44(55-31-38-24-14-5-15-25-38)43(54-30-37-22-12-4-13-23-37)42(53-29-36-20-10-3-11-21-36)41(57-46)33-52-28-35-18-8-2-9-19-35/h1-25,27,40-44,46H,26,28-33H2,(H,50,51)/t40-,41+,42+,43-,44+,46+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human SHP2 by pNPP hydrolysis assay |
Bioorg Med Chem Lett 21: 1092-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.126
BindingDB Entry DOI: 10.7270/Q2D79BP8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data