

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268509 (CHEMBL495623 | N-[3-(3-(3,4-Dichlorophenoxy)phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268510 (CHEMBL495624 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268511 (CHEMBL497634 | N-[3-(3-(4-Chlorophenoxy)phenyl)pro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268512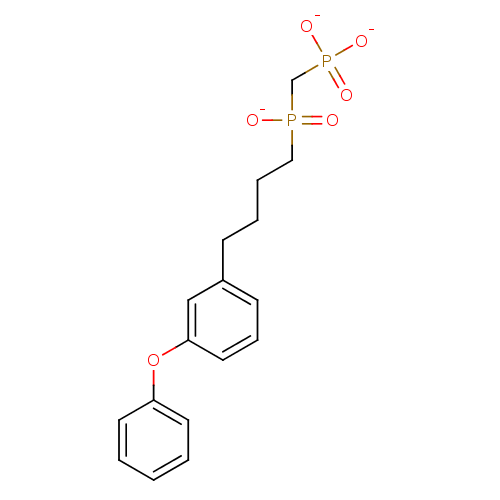 (3-(3-Phenoxyphenyl)propylphosphinylmethylphosphoni...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268627 (CHEMBL496801 | N-Hydroxy-2-phosphono-5-(3-phenoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268562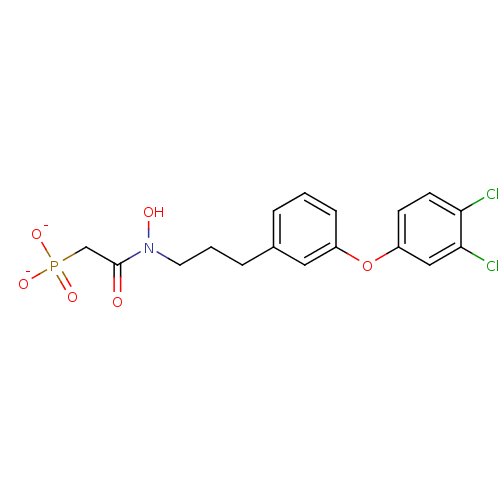 (CHEMBL497616 | N-Hydroxy-N-[3-(3-(3,4-dichlorophen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268563 (CHEMBL497617 | N-Hydroxy-N-[3-(3-phenoxyphenyl)pro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268625 (CHEMBL447414 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268510 (CHEMBL495624 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268509 (CHEMBL495623 | N-[3-(3-(3,4-Dichlorophenoxy)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268564 (CHEMBL497815 | N-[3-(4-Biphenyl)propyl]phosphonoac...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268565 (CHEMBL497410 | N-[3-(3-Phenoxyphenyl)propyl]sulfoa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268566 (CHEMBL497618 | N-Methyl-N-[3-(3-phenoxyphenyl)prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268625 (CHEMBL447414 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268566 (CHEMBL497618 | N-Methyl-N-[3-(3-phenoxyphenyl)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268511 (CHEMBL497634 | N-[3-(3-(4-Chlorophenoxy)phenyl)pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268626 (CHEMBL524084 | N-[2-(3-Phenoxyphenyl)ethyl]phospho...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268562 (CHEMBL497616 | N-Hydroxy-N-[3-(3-(3,4-dichlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268627 (CHEMBL496801 | N-Hydroxy-2-phosphono-5-(3-phenoxyp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268628 (CHEMBL496802 | N-[4-(3-Phenoxyphenyl)butyl]phospho...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268629 (3-(3-Phenoxyphenyl)propyl Phosphonoacetate Dipotas...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268626 (CHEMBL524084 | N-[2-(3-Phenoxyphenyl)ethyl]phospho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268678 (CHEMBL525377 | N-Hydroxy-N-[3-(4-methylbiphenyl)pr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268679 (CHEMBL523897 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268677 (CHEMBL498628 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50268676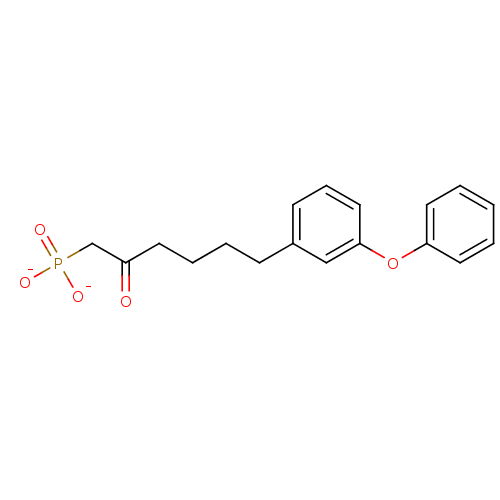 (2-Oxo-6-(4-phenoxyphenyl)hexylphosphonic Acid Dipo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus histidine tagged dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectropho... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268676 (2-Oxo-6-(4-phenoxyphenyl)hexylphosphonic Acid Dipo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268628 (CHEMBL496802 | N-[4-(3-Phenoxyphenyl)butyl]phospho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268565 (CHEMBL497410 | N-[3-(3-Phenoxyphenyl)propyl]sulfoa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268564 (CHEMBL497815 | N-[3-(4-Biphenyl)propyl]phosphonoac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268679 (CHEMBL523897 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268512 (3-(3-Phenoxyphenyl)propylphosphinylmethylphosphoni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268677 (CHEMBL498628 | N-[3-(3-Phenoxyphenyl)propyl]phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268678 (CHEMBL525377 | N-Hydroxy-N-[3-(4-methylbiphenyl)pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268629 (3-(3-Phenoxyphenyl)propyl Phosphonoacetate Dipotas...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50268563 (CHEMBL497617 | N-Hydroxy-N-[3-(3-phenoxyphenyl)pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli cells assessed as conversion of [3H]FPP to squalene by liquid scintil... | J Med Chem 52: 3869-80 (2009) Article DOI: 10.1021/jm9001764 BindingDB Entry DOI: 10.7270/Q2XK8FF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467352 (CHEMBL4294193) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.124 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of CXCR4 tropic HIV1 3B reverse transcriptase infected in human TZM-bl cells | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467341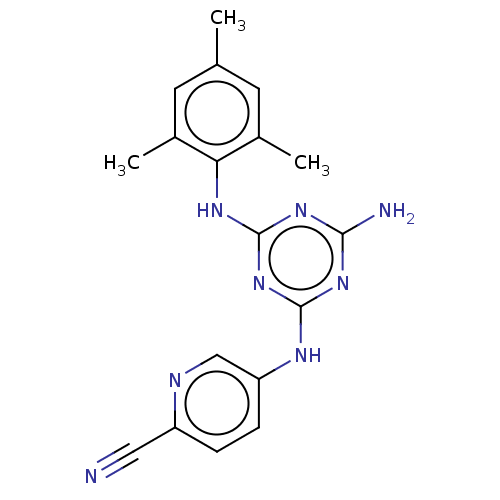 (CHEMBL4294624) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.131 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of CXCR4 tropic HIV1 3B reverse transcriptase infected in human TZM-bl cells | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467341 (CHEMBL4294624) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of CCR5 tropic HIV1 VB59 reverse transcriptase infected in human TZM-bl cells | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467341 (CHEMBL4294624) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of CCR5 tropic HIV1 ADA5 reverse transcriptase infected in human TZM-bl cells | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467352 (CHEMBL4294193) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.273 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of CCR5 tropic HIV1 ADA5 reverse transcriptase infected in human TZM-bl cells | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467332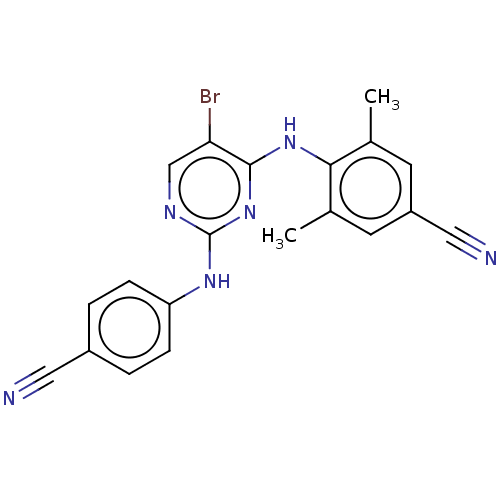 (CHEMBL70517) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HIV1 LAI reverse transcriptase infected in human MT4 cells assessed as protection against virus-induced cytopathic effect by MTT assay | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467332 (CHEMBL70517) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant infected in human MT4 cells assessed as protection against virus-induced cytopathic effect by M... | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467349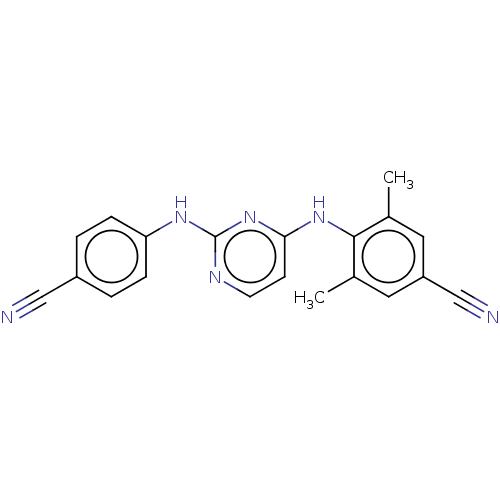 (CHEMBL70442) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HIV1 LAI reverse transcriptase infected in human MT4 cells assessed as protection against virus-induced cytopathic effect by MTT assay | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467352 (CHEMBL4294193) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.497 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of CCR5 tropic HIV1 VB59 reverse transcriptase infected in human TZM-bl cells | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467326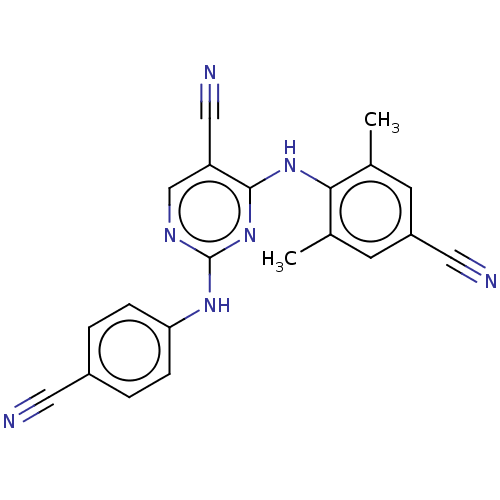 (CHEMBL69890) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HIV1 LAI reverse transcriptase infected in human MT4 cells assessed as protection against virus-induced cytopathic effect by MTT assay | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467341 (CHEMBL4294624) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.784 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of CXCR4 tropic HIV1 UG070 reverse transcriptase infected in human TZM-bl cells | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467345 (CHEMBL308004) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HIV1 LAI reverse transcriptase infected in human MT4 cells assessed as protection against virus-induced cytopathic effect by MTT assay | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467337 (CHEMBL312764) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N mutant infected in human MT4 cells assessed as protection against virus-induced cytopathic effect by M... | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50467329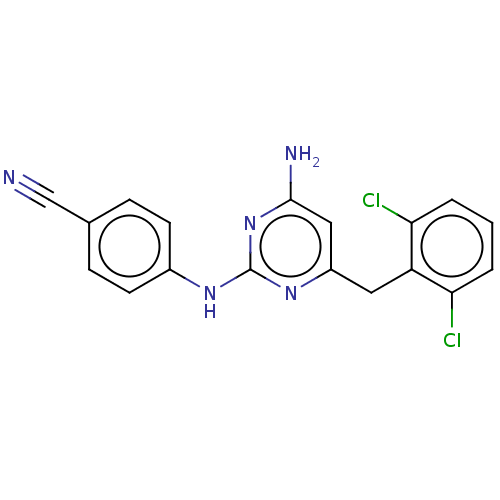 (CHEMBL74239) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of HIV1 LAI reverse transcriptase infected in human MT4 cells assessed as protection against virus-induced cytopathic effect by MTT assay | Eur J Med Chem 158: 371-392 (2018) Article DOI: 10.1016/j.ejmech.2018.09.013 BindingDB Entry DOI: 10.7270/Q2V98BRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 249 total ) | Next | Last >> |