 Found 40 hits with Last Name = 'furuichi' and Initial = 'h'
Found 40 hits with Last Name = 'furuichi' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465744
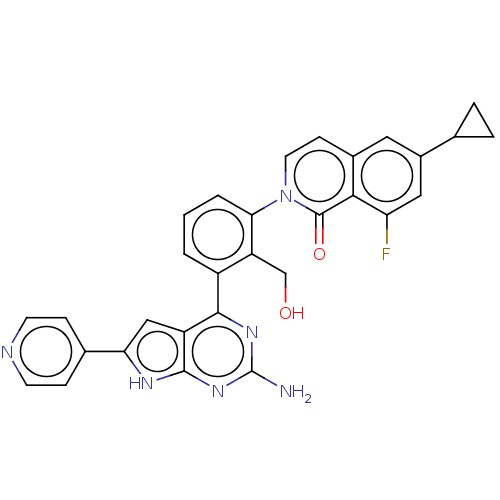
(2-{3-[2-amino-6-(pyridin-4-yl)-7H- pyrrolo[2,3-d]p...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)-c1ccncc1 Show InChI InChI=1S/C30H23FN6O2/c31-23-13-19(16-4-5-16)12-18-8-11-37(29(39)26(18)23)25-3-1-2-20(22(25)15-38)27-21-14-24(17-6-9-33-10-7-17)34-28(21)36-30(32)35-27/h1-3,6-14,16,38H,4-5,15H2,(H3,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468316
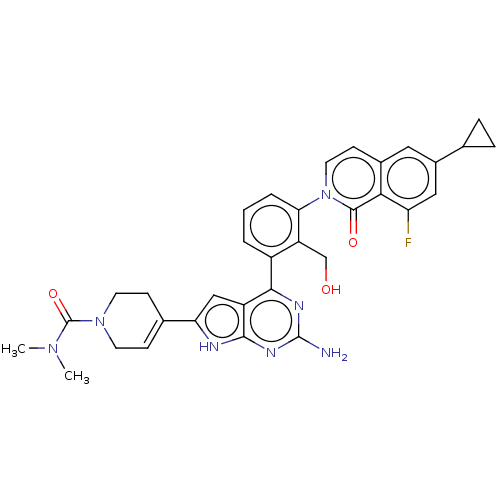
(4-{2-amino-4-[3- (6-cyclopropyl-8- fluoro-1-oxoiso...)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 |c:8| Show InChI InChI=1S/C33H32FN7O3/c1-39(2)33(44)40-11-8-19(9-12-40)26-16-23-29(37-32(35)38-30(23)36-26)22-4-3-5-27(24(22)17-42)41-13-10-20-14-21(18-6-7-18)15-25(34)28(20)31(41)43/h3-5,8,10,13-16,18,42H,6-7,9,11-12,17H2,1-2H3,(H3,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468313
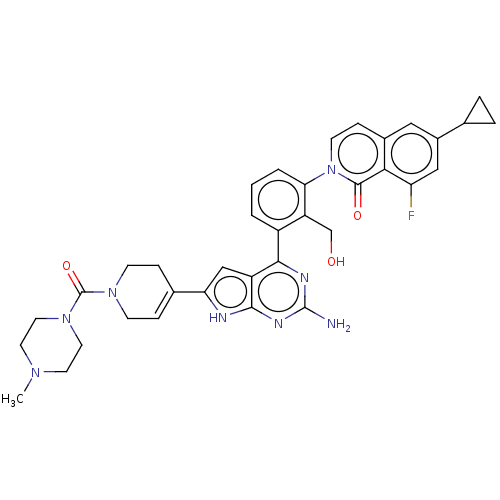
(2-(3-{2-amino-6- [1-(4-methylpiperazine-1- carbony...)Show SMILES CN1CCN(CC1)C(=O)N1CCC(=CC1)c1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 |c:13| Show InChI InChI=1S/C36H37FN8O3/c1-42-13-15-44(16-14-42)36(48)43-10-7-22(8-11-43)29-19-26-32(40-35(38)41-33(26)39-29)25-3-2-4-30(27(25)20-46)45-12-9-23-17-24(21-5-6-21)18-28(37)31(23)34(45)47/h2-4,7,9,12,17-19,21,46H,5-6,8,10-11,13-16,20H2,1H3,(H3,38,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK C481S mutant using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468313

(2-(3-{2-amino-6- [1-(4-methylpiperazine-1- carbony...)Show SMILES CN1CCN(CC1)C(=O)N1CCC(=CC1)c1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 |c:13| Show InChI InChI=1S/C36H37FN8O3/c1-42-13-15-44(16-14-42)36(48)43-10-7-22(8-11-43)29-19-26-32(40-35(38)41-33(26)39-29)25-3-2-4-30(27(25)20-46)45-12-9-23-17-24(21-5-6-21)18-28(37)31(23)34(45)47/h2-4,7,9,12,17-19,21,46H,5-6,8,10-11,13-16,20H2,1H3,(H3,38,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468320
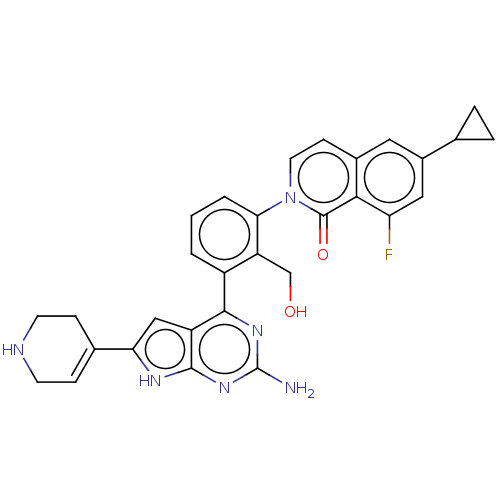
(2-{3-[2-amino-6-(1,2,3,6- tetrahydropyridin-4-yl)-...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCNCC1 |t:39| Show InChI InChI=1S/C30H27FN6O2/c31-23-13-19(16-4-5-16)12-18-8-11-37(29(39)26(18)23)25-3-1-2-20(22(25)15-38)27-21-14-24(17-6-9-33-10-7-17)34-28(21)36-30(32)35-27/h1-3,6,8,11-14,16,33,38H,4-5,7,9-10,15H2,(H3,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50586595
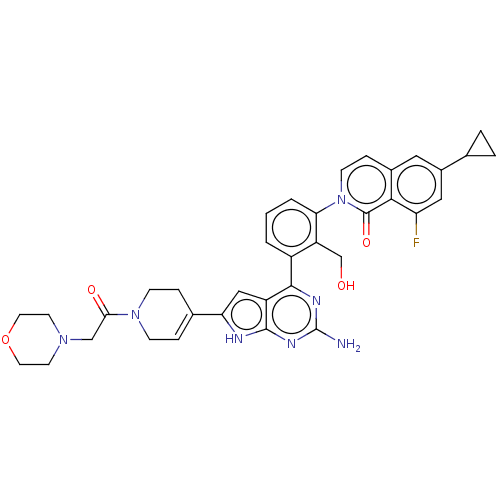
(CHEMBL5083974)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C(=O)CN1CCOCC1 |t:39| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50586593
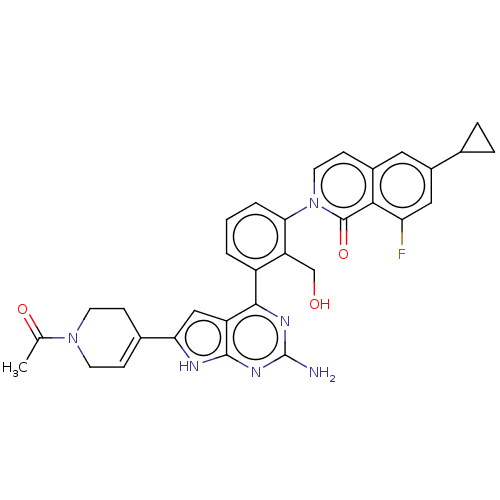
(CHEMBL5076374)Show SMILES CC(=O)N1CCC(=CC1)c1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 |c:6| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468316

(4-{2-amino-4-[3- (6-cyclopropyl-8- fluoro-1-oxoiso...)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 |c:8| Show InChI InChI=1S/C33H32FN7O3/c1-39(2)33(44)40-11-8-19(9-12-40)26-16-23-29(37-32(35)38-30(23)36-26)22-4-3-5-27(24(22)17-42)41-13-10-20-14-21(18-6-7-18)15-25(34)28(20)31(41)43/h3-5,8,10,13-16,18,42H,6-7,9,11-12,17H2,1-2H3,(H3,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK C481S mutant using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK C481S mutant using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468320

(2-{3-[2-amino-6-(1,2,3,6- tetrahydropyridin-4-yl)-...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCNCC1 |t:39| Show InChI InChI=1S/C30H27FN6O2/c31-23-13-19(16-4-5-16)12-18-8-11-37(29(39)26(18)23)25-3-1-2-20(22(25)15-38)27-21-14-24(17-6-9-33-10-7-17)34-28(21)36-30(32)35-27/h1-3,6,8,11-14,16,33,38H,4-5,7,9-10,15H2,(H3,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK C481S mutant using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human Ramos cells assessed as reduction of IgM stimulated PLCgamma2 (Y1217) phosphorylation after 24 hrs by western blot assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type BTK (unknown origin) in presence of 1 mM ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human Ramos cells assessed as reduction in BTK phosphorylation at Tyr223 residue incubated for 24 hrs by Western blot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TEC (359 - 631 residues) expressed in baculovirus expression system by mobility shift assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human Ramos cells assessed as reduction in BTK phosphorylation at Tyr223 residue incubated for 24 hrs by Western blot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human Ramos cells assessed as reduction of IgM stimulated PLCgamma2 (Y1217) phosphorylation after 24 hrs by western blot assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465742
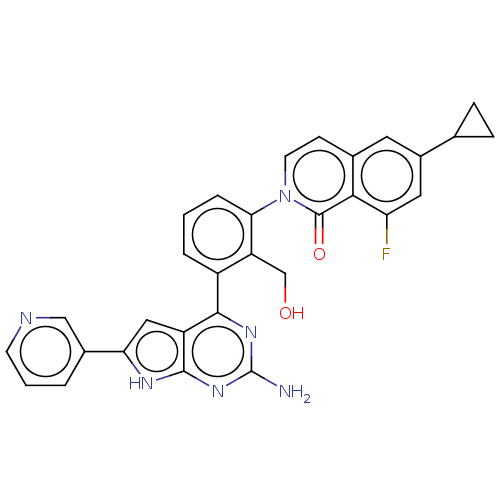
(2-{3-[2-amino-6-(pyridin-3-yl)-7H- pyrrolo[2,3-d]p...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)-c1cccnc1 Show InChI InChI=1S/C30H23FN6O2/c31-23-12-19(16-6-7-16)11-17-8-10-37(29(39)26(17)23)25-5-1-4-20(22(25)15-38)27-21-13-24(18-3-2-9-33-14-18)34-28(21)36-30(32)35-27/h1-5,8-14,16,38H,6-7,15H2,(H3,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50586594
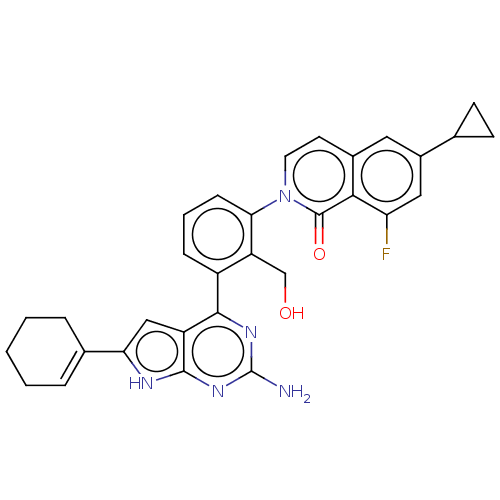
(CHEMBL5082144)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCCCC1 |t:39| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type BTK (unknown origin) in presence of 1 mM ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK C481S mutant (unknown origin) in presence of 1 mM ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human whole blood assessed as activation of anti-IgM-induced CD69 expression by flow cytometry analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full-length BMX (1 to 675 residues) expressed in baculovirus expression system by mobility shift assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human whole blood assessed as activation of anti-IgM-induced CD69 expression by flow cytometry analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK C481S mutant (unknown origin) in presence of 1 mM ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465726

(2-[3-(2-amino-6-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)-c1ccccc1 Show InChI InChI=1S/C31H24FN5O2/c32-24-14-20(17-9-10-17)13-19-11-12-37(30(39)27(19)24)26-8-4-7-21(23(26)16-38)28-22-15-25(18-5-2-1-3-6-18)34-29(22)36-31(33)35-28/h1-8,11-15,17,38H,9-10,16H2,(H3,33,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465732
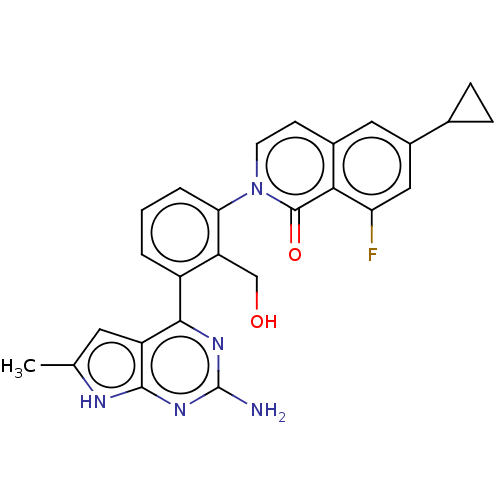
(2-[3-(2-amino-6-methyl- 7H-pyrrolo[2,3- d]pyrimidi...)Show SMILES Cc1cc2c(nc(N)nc2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 Show InChI InChI=1S/C26H22FN5O2/c1-13-9-18-23(30-26(28)31-24(18)29-13)17-3-2-4-21(19(17)12-33)32-8-7-15-10-16(14-5-6-14)11-20(27)22(15)25(32)34/h2-4,7-11,14,33H,5-6,12H2,1H3,(H3,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM465731
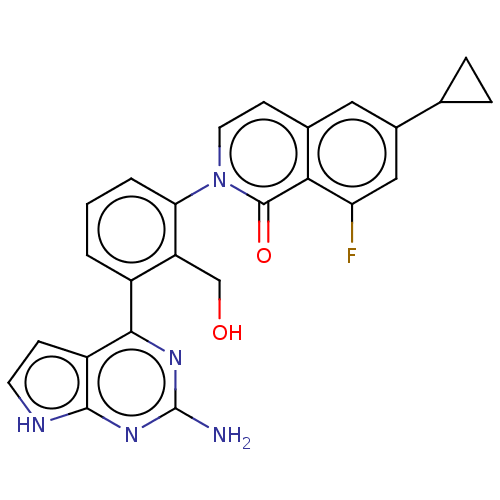
(2-[3-(2-amino-7H-pyrrolo[2,3- d]pyrimidin-4-yl)-2-...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc[nH]c2n1 Show InChI InChI=1S/C25H20FN5O2/c26-19-11-15(13-4-5-13)10-14-7-9-31(24(33)21(14)19)20-3-1-2-16(18(20)12-32)22-17-6-8-28-23(17)30-25(27)29-22/h1-3,6-11,13,32H,4-5,12H2,(H3,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK-A using phosphorylated substrate in presence of ATP by microplate reader assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ITK (2 - 620 residues) expressed in baculovirus expression system by mobility shift assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDR1 (444 - 876 residues) expressed in baculovirus expression system by mobility shift assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length SRC expressed in baculovirus expression system by mobility shift assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Muscle, skeletal receptor tyrosine-protein kinase
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 306 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MUSK expressed in baculovirus expression system by mobility shift assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2B6 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2CD6 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM465748

(2-(3-{2-amino-6-[1-(oxetan-3-yl)-1,2,3,6-tetrahydr...)Show SMILES Nc1nc(-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)c2cc([nH]c2n1)C1=CCN(CC1)C1COC1 |t:39| Show InChI InChI=1S/C33H31FN6O3/c34-26-13-21(18-4-5-18)12-20-8-11-40(32(42)29(20)26)28-3-1-2-23(25(28)15-41)30-24-14-27(36-31(24)38-33(35)37-30)19-6-9-39(10-7-19)22-16-43-17-22/h1-3,6,8,11-14,18,22,41H,4-5,7,9-10,15-17H2,(H3,35,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01279
BindingDB Entry DOI: 10.7270/Q2SB49NZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data