

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50192018 (CHEMBL3350037) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity for SSTR2 receptors of rat cortex membranes was determined by using [125I][Tyr3]-octreotide radioligand | Bioorg Med Chem Lett 8: 1207-10 (1999) BindingDB Entry DOI: 10.7270/Q2DB834V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50215551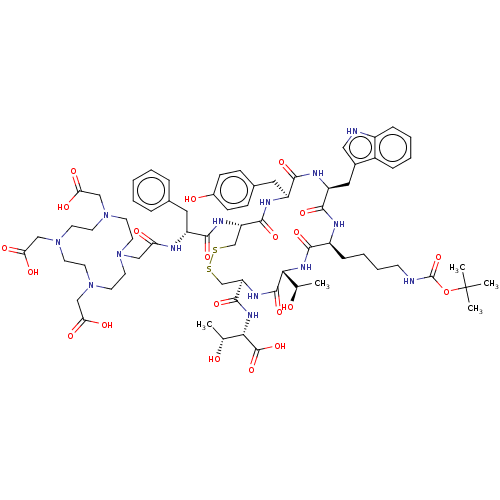 (CHEMBL410194) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity for SSTR2 receptors of rat cortex membranes was determined by using [125I][Tyr3]-octreotide radioligand | Bioorg Med Chem Lett 8: 1207-10 (1999) BindingDB Entry DOI: 10.7270/Q2DB834V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50215550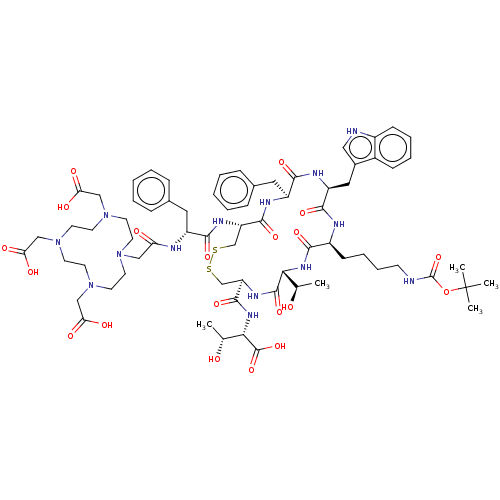 (CHEMBL407643) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity for SSTR2 receptors of rat cortex membranes was determined by using [125I][Tyr3]-octreotide radioligand | Bioorg Med Chem Lett 8: 1207-10 (1999) BindingDB Entry DOI: 10.7270/Q2DB834V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097967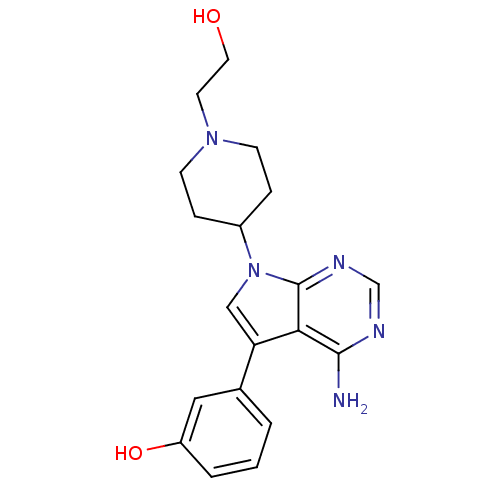 (3-(4-amino-7-(1-(2-hydroxyethyl)piperidin-4-yl)-7H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50192018 (CHEMBL3350037) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Binding affinity for SSTR2 receptors of rat cortex membranes was determined by using Y-labelled SMT487 radioligand | Bioorg Med Chem Lett 8: 1207-10 (1999) BindingDB Entry DOI: 10.7270/Q2DB834V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097971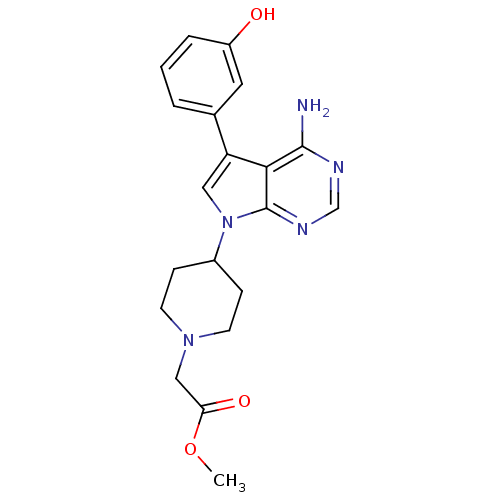 (CHEMBL262276 | methyl 2-(4-(4-amino-5-(3-hydroxyph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Concentration required for inhibition of v-Abl receptor by tyrosine kinase enzyme assay | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097979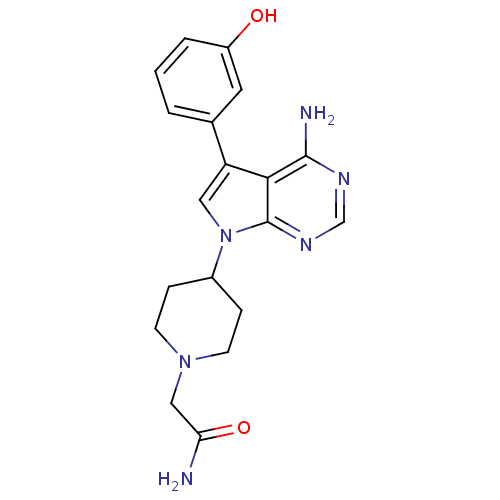 (2-(4-(4-amino-5-(3-hydroxyphenyl)-7H-pyrrolo[2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097958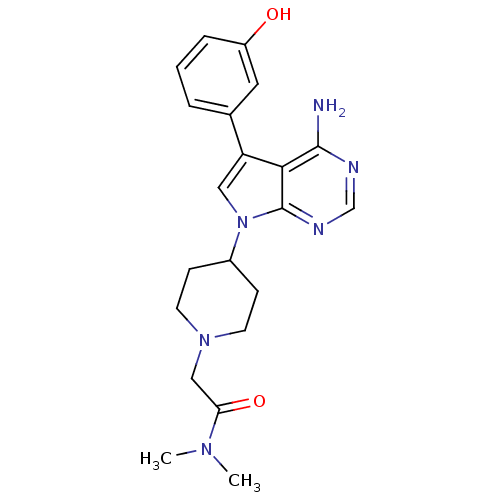 (2-(4-(4-amino-5-(3-hydroxyphenyl)-7H-pyrrolo[2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097991 (CHEMBL355019 | {3-[4-Amino-5-(3-hydroxy-phenyl)-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097962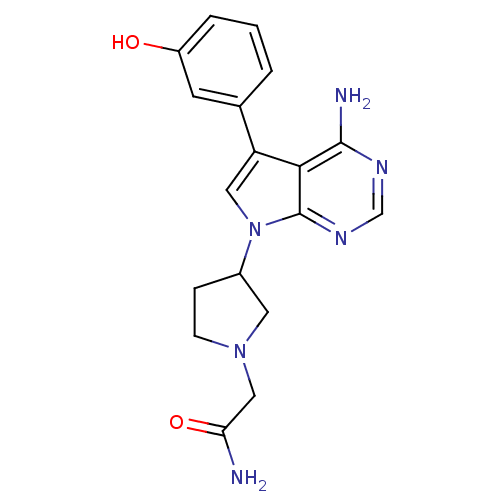 (2-{3-[4-Amino-5-(3-hydroxy-phenyl)-pyrrolo[2,3-d]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097968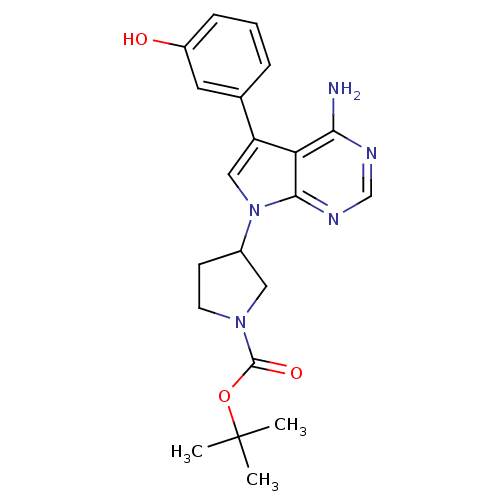 (3-[4-Amino-5-(3-hydroxy-phenyl)-pyrrolo[2,3-d]pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097986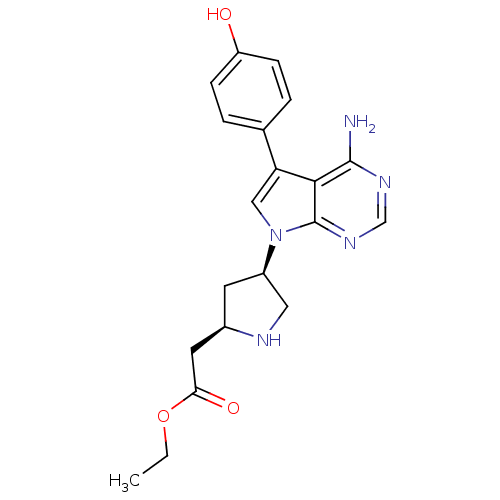 (CHEMBL169939 | {(R)-4-[(S)-4-Amino-5-(4-hydroxy-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097974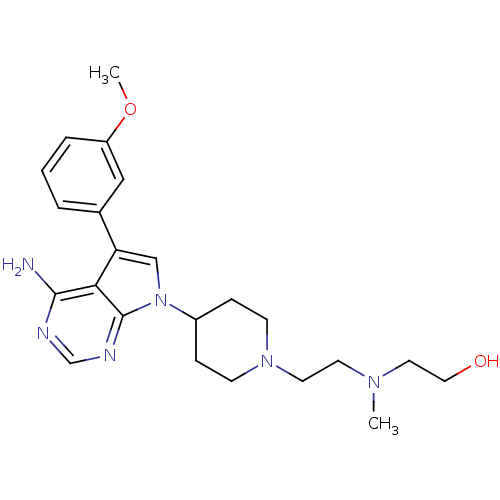 (2-((2-(4-(4-amino-5-(3-methoxyphenyl)-7H-pyrrolo[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097972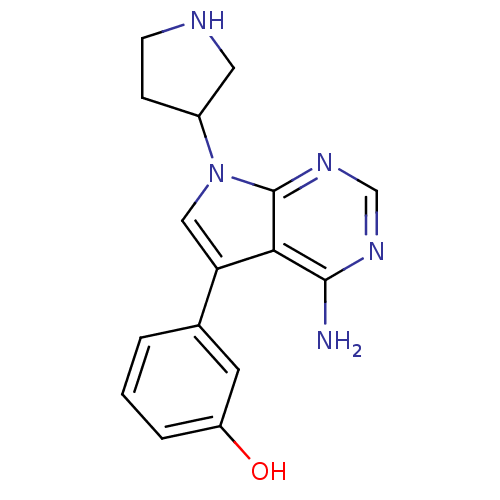 (3-(4-Amino-7-pyrrolidin-3-yl-7H-pyrrolo[2,3-d]pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097970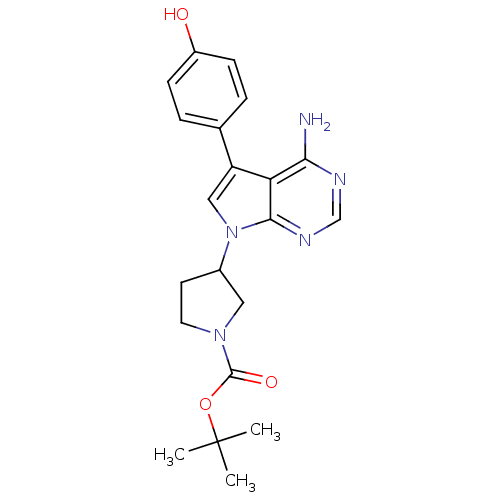 (3-[4-Amino-5-(4-hydroxy-phenyl)-pyrrolo[2,3-d]pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097959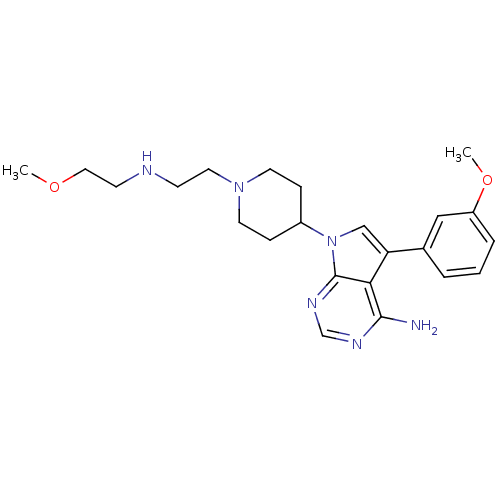 (7-{1-[2-(2-Methoxy-ethylamino)-ethyl]-piperidin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097976 (CHEMBL352829 | [(R)-4-((S)-4-Amino-5-phenyl-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097964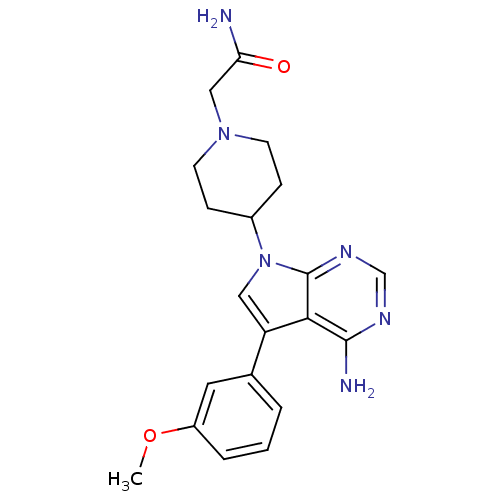 (2-(4-(4-amino-5-(3-methoxyphenyl)-7H-pyrrolo[2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097965 ((R)-4-[(R)-4-Amino-5-(4-hydroxy-phenyl)-pyrrolo[2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097973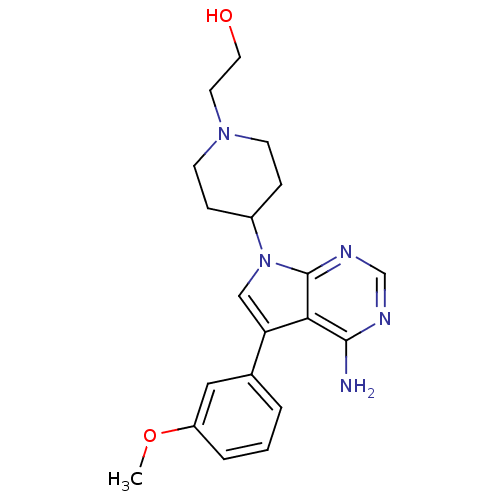 (2-(4-(4-amino-5-(3-methoxyphenyl)-7H-pyrrolo[2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097988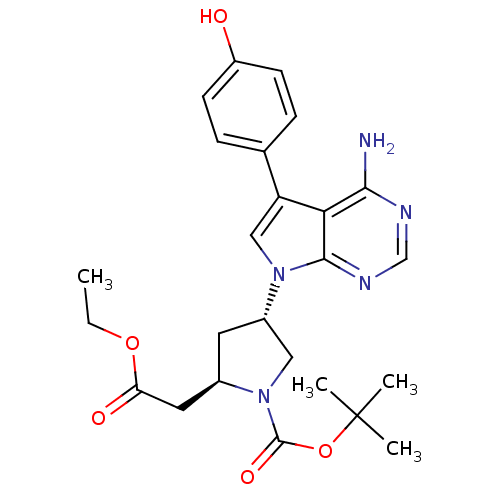 ((R)-4-[(R)-4-Amino-5-(4-hydroxy-phenyl)-pyrrolo[2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097978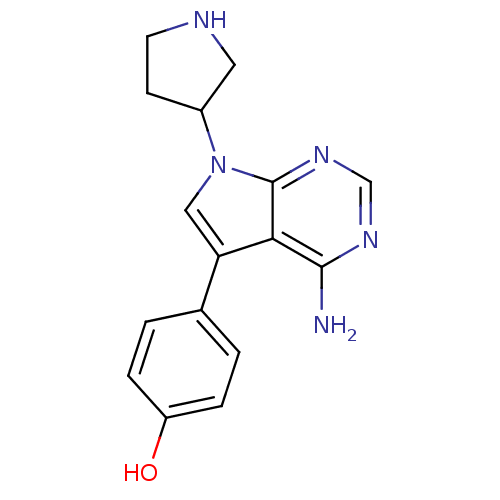 (4-(4-Amino-7-pyrrolidin-3-yl-7H-pyrrolo[2,3-d]pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097960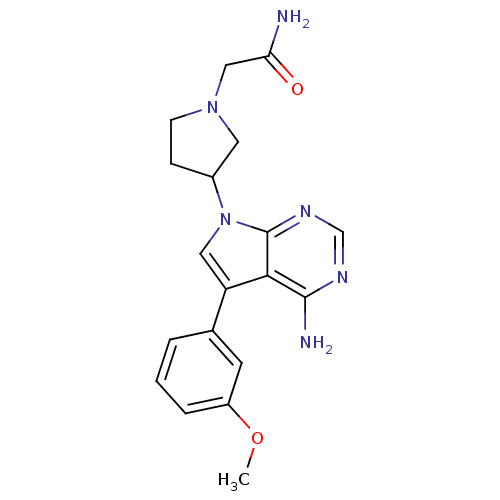 (2-{3-[4-Amino-5-(3-methoxy-phenyl)-pyrrolo[2,3-d]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097963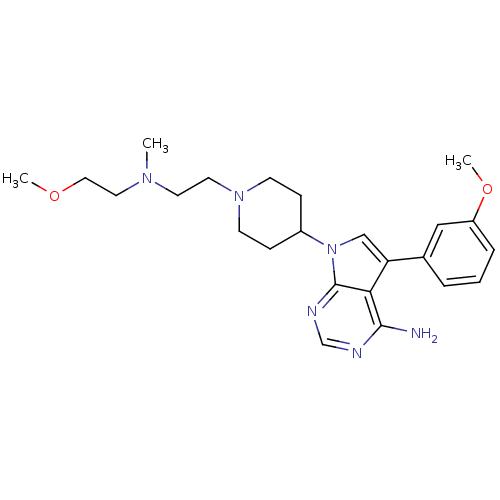 (7-(1-(2-((2-methoxyethyl)(methyl)amino)ethyl)piper...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097982 (4-[(S)-4-Amino-7-((R)-5-hydroxymethyl-pyrrolidin-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097989 (CHEMBL355891 | {3-[4-Amino-5-(3-methoxy-phenyl)-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097987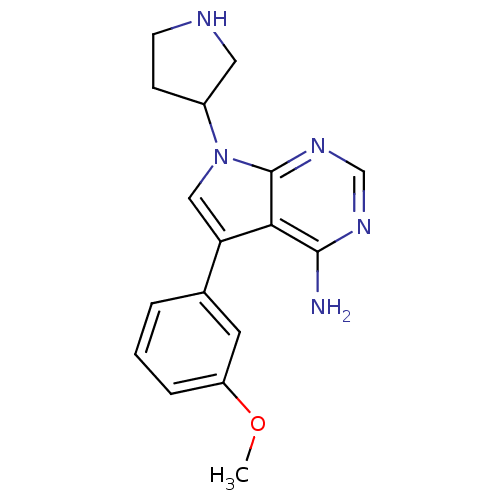 (5-(3-Methoxy-phenyl)-7-pyrrolidin-3-yl-7H-pyrrolo[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1/ABL2 (Homo sapiens (Human)) | BDBM50097991 (CHEMBL355019 | {3-[4-Amino-5-(3-hydroxy-phenyl)-py...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Concentration required for inhibition of v-Abl receptor by tyrosine kinase enzyme assay | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1/ABL2 (Homo sapiens (Human)) | BDBM50097971 (CHEMBL262276 | methyl 2-(4-(4-amino-5-(3-hydroxyph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Concentration required for inhibition of v-Abl receptor by tyrosine kinase enzyme assay | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1/ABL2 (Homo sapiens (Human)) | BDBM50097968 (3-[4-Amino-5-(3-hydroxy-phenyl)-pyrrolo[2,3-d]pyri...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Concentration required for inhibition of v-Abl receptor by tyrosine kinase enzyme assay | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097961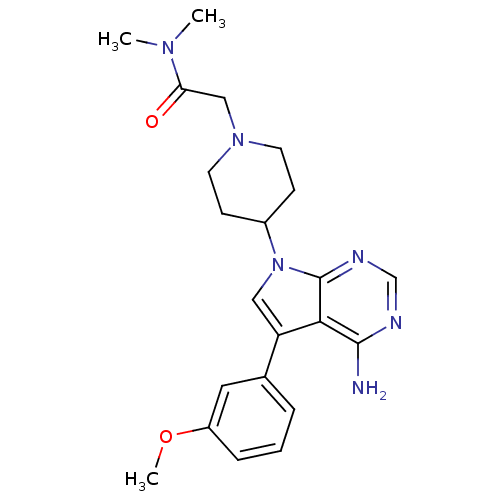 (2-(4-(4-amino-5-(3-methoxyphenyl)-7H-pyrrolo[2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097980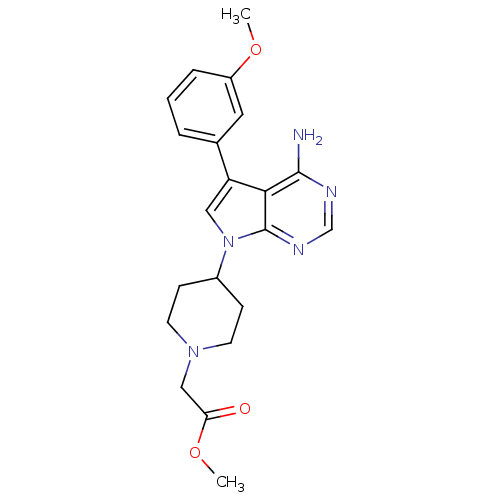 (CHEMBL169065 | methyl 2-(4-(4-amino-5-(3-methoxyph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097992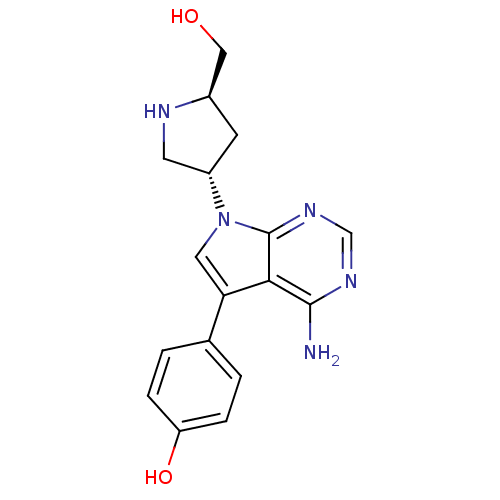 (4-[(R)-4-Amino-7-((R)-5-hydroxymethyl-pyrrolidin-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1/ABL2 (Homo sapiens (Human)) | BDBM50097967 (3-(4-amino-7-(1-(2-hydroxyethyl)piperidin-4-yl)-7H...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Concentration required for inhibition of epidermal growth factor receptor (EGF-R) by tyrosine kinase enzyme activity | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097998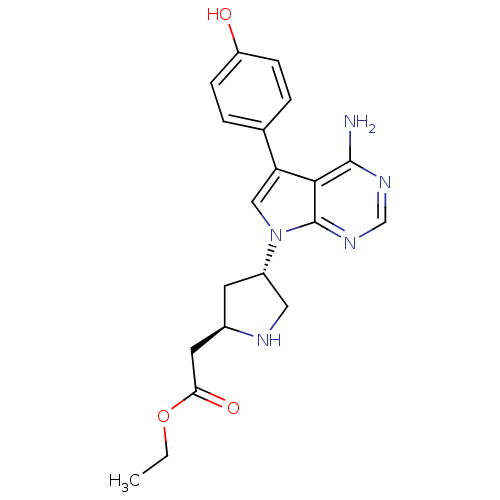 (CHEMBL170726 | {(R)-4-[(R)-4-Amino-5-(4-hydroxy-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1/ABL2 (Homo sapiens (Human)) | BDBM50097958 (2-(4-(4-amino-5-(3-hydroxyphenyl)-7H-pyrrolo[2,3-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of v-Abl receptor tyrosine kinase enzyme activity | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097981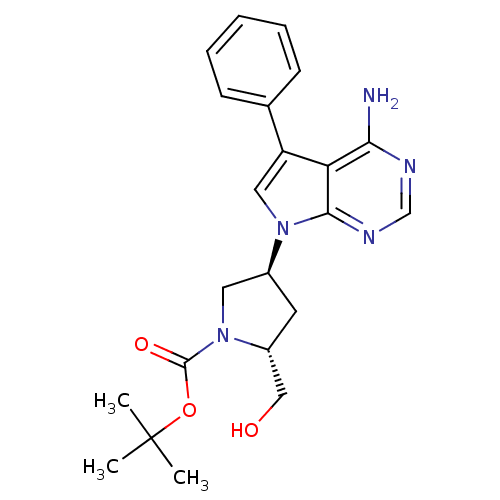 ((R)-4-((R)-4-Amino-5-phenyl-pyrrolo[2,3-d]pyrimidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097996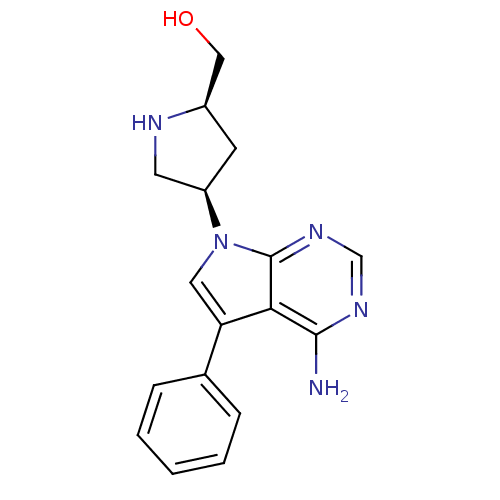 (CHEMBL168560 | [(R)-4-((S)-4-Amino-5-phenyl-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1/ABL2 (Homo sapiens (Human)) | BDBM50097962 (2-{3-[4-Amino-5-(3-hydroxy-phenyl)-pyrrolo[2,3-d]p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of epidermal growth factor receptor (EGF-R) in a cellular assay | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50097979 (2-(4-(4-amino-5-(3-hydroxyphenyl)-7H-pyrrolo[2,3-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Concentration required for inhibition of epidermal growth factor receptor (EGF-R) by tyrosine kinase enzyme activity | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097969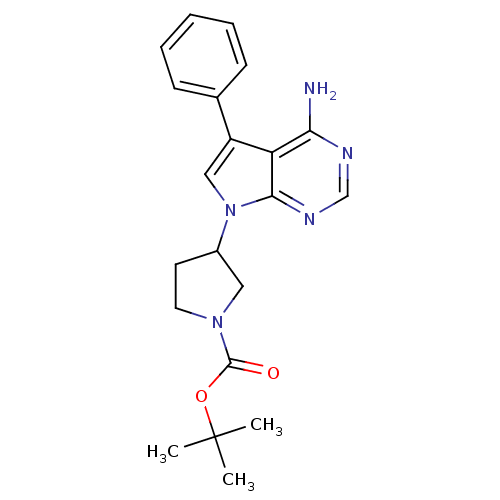 (3-(4-Amino-5-phenyl-pyrrolo[2,3-d]pyrimidin-7-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50097979 (2-(4-(4-amino-5-(3-hydroxyphenyl)-7H-pyrrolo[2,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Tested for inhibitory activity against Epidermal growth factor receptor | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM14712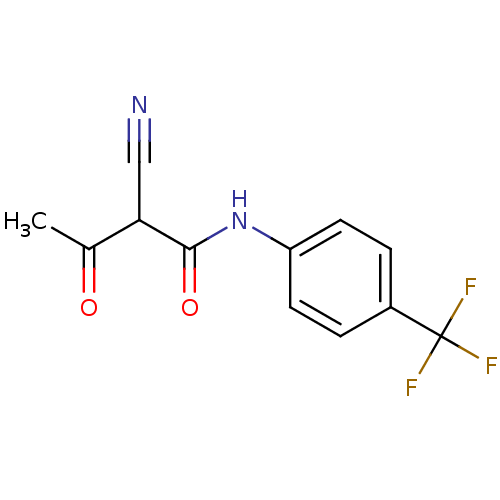 ((2Z)-2-cyano-3-hydroxy-N-[4-(trifluoromethyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Immunosuppressive activity of the compound expressed as ability to inhibit human recombinant dihydroorotate dehydrogenase (DHODH) | Bioorg Med Chem Lett 8: 2203-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN65X4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1/ABL2 (Homo sapiens (Human)) | BDBM50097979 (2-(4-(4-amino-5-(3-hydroxyphenyl)-7H-pyrrolo[2,3-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of autophosphorylation of epidermal growth factor receptor (EGF-R) in a cellular assay | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097995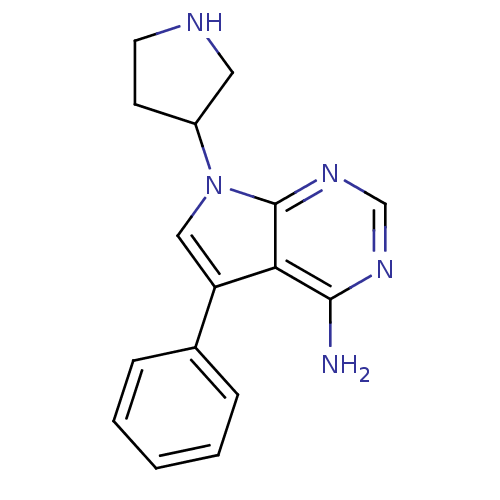 (5-Phenyl-7-pyrrolidin-3-yl-7H-pyrrolo[2,3-d]pyrimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1/ABL2 (Homo sapiens (Human)) | BDBM50097959 (7-{1-[2-(2-Methoxy-ethylamino)-ethyl]-piperidin-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Concentration required for inhibition of v-Abl receptor by tyrosine kinase enzyme assay | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50097966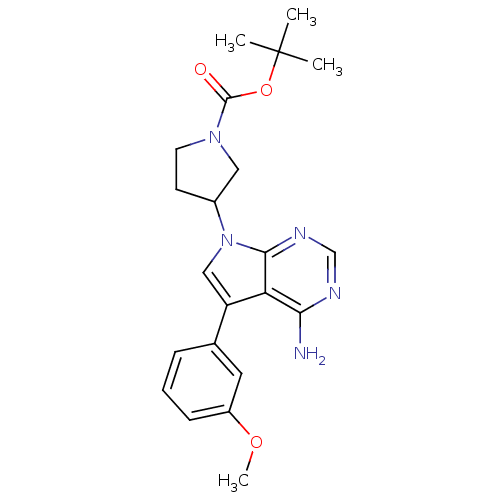 (3-[4-Amino-5-(3-methoxy-phenyl)-pyrrolo[2,3-d]pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Inhibition of p60 c-Src tyrosine kinase enzyme activity in liquid-phase tyrosine phosphorylation assay at 830 ng/mL | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50097971 (CHEMBL262276 | methyl 2-(4-(4-amino-5-(3-hydroxyph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Tested for inhibitory activity against Epidermal growth factor receptor | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50097959 (7-{1-[2-(2-Methoxy-ethylamino)-ethyl]-piperidin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Concentration required for inhibition of c-Src mediated phosphorylation of Fak in IC8.1 fibroblast cell assay | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1/ABL2 (Homo sapiens (Human)) | BDBM50097972 (3-(4-Amino-7-pyrrolidin-3-yl-7H-pyrrolo[2,3-d]pyri...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma Research Curated by ChEMBL | Assay Description Concentration required for inhibition of v-Abl receptor by tyrosine kinase enzyme assay | Bioorg Med Chem Lett 11: 853-6 (2001) BindingDB Entry DOI: 10.7270/Q29P30XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 181 total ) | Next | Last >> |