

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059767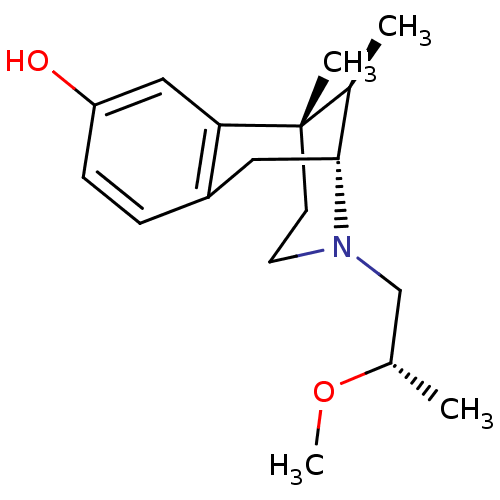 ((2R,6S,11R)-3-((S)-2-Methoxy-propyl)-6,11-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059778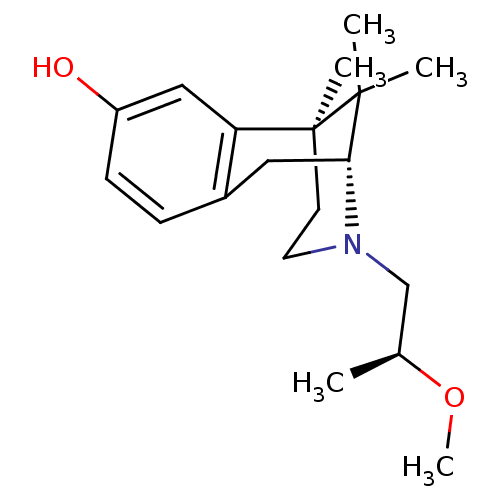 ((2R,6S)-3-((S)-2-Methoxy-propyl)-6,11,11-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059761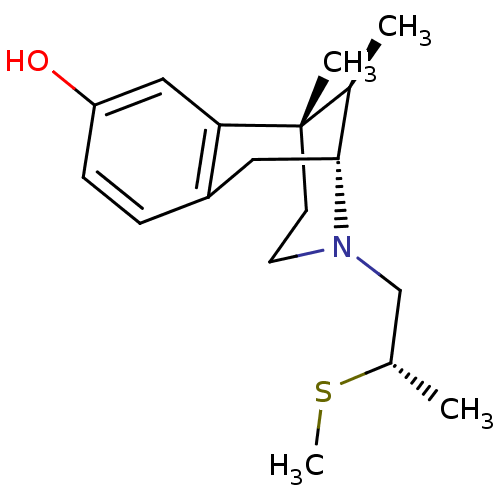 ((2R,6S,11R)-6,11-Dimethyl-3-((S)-2-methylsulfanyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059772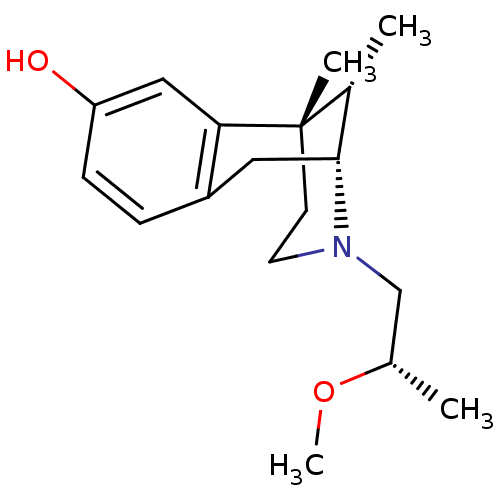 ((2R,6S,11S)-3-((S)-2-Methoxy-propyl)-6,11-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059764 ((2R,6S,11R)-3-(2-Methoxy-2-methyl-propyl)-6,11-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059786 ((2R,6S,11R)-6,11-Dimethyl-3-((R)-2-methylsulfanyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059800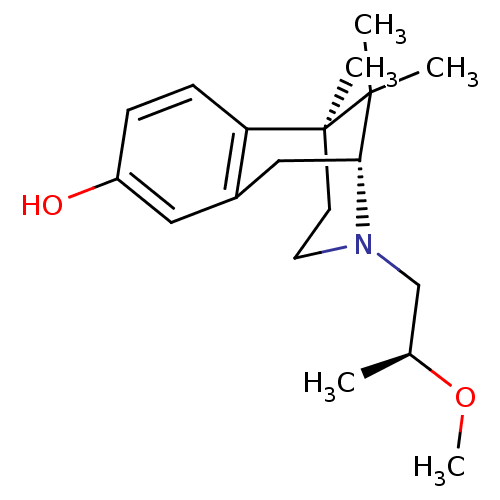 ((2R,6S)-3-((S)-2-Methoxy-propyl)-6,11,11-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059794 ((2R,6R)-3-((S)-2-Methoxy-propyl)-6,11,11-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059803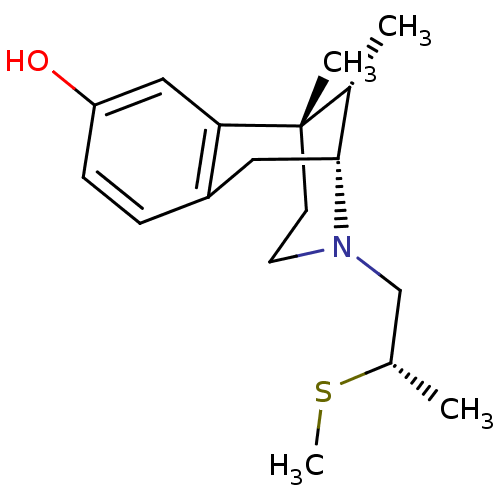 ((2R,6S,11S)-6,11-Dimethyl-3-((S)-2-methylsulfanyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059783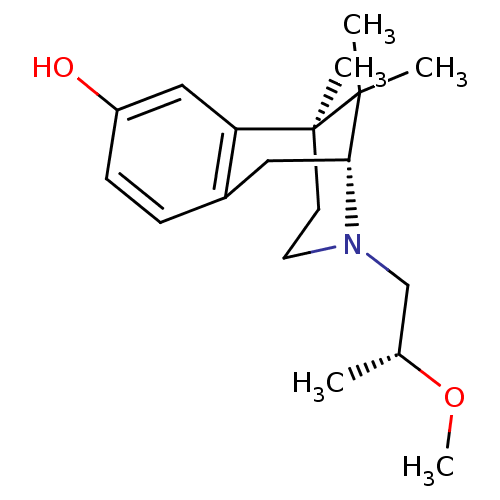 ((2R,6S)-3-((R)-2-Methoxy-propyl)-6,11,11-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059788 ((2R,6S)-3-((S)-2-Methoxy-propyl)-6,11,11-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059766 ((2R,6S,11R)-3-((R)-2-Methoxy-propyl)-6,11-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059792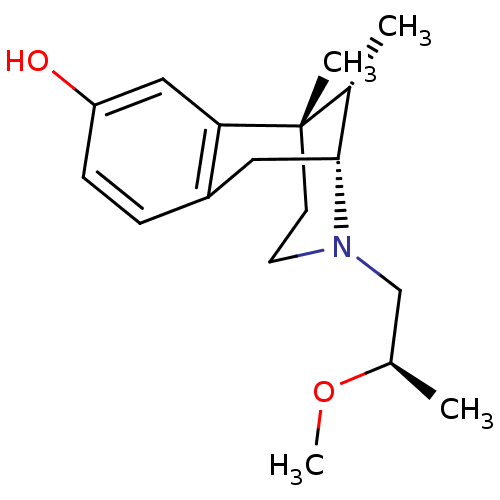 ((2R,6S,11S)-3-((R)-2-Methoxy-propyl)-6,11-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059797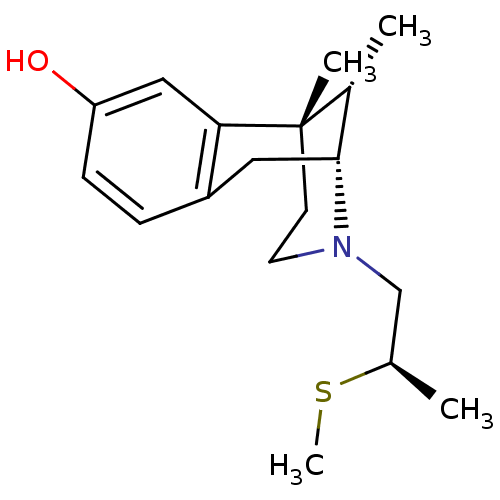 ((2R,6S,11S)-6,11-Dimethyl-3-((R)-2-methylsulfanyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059771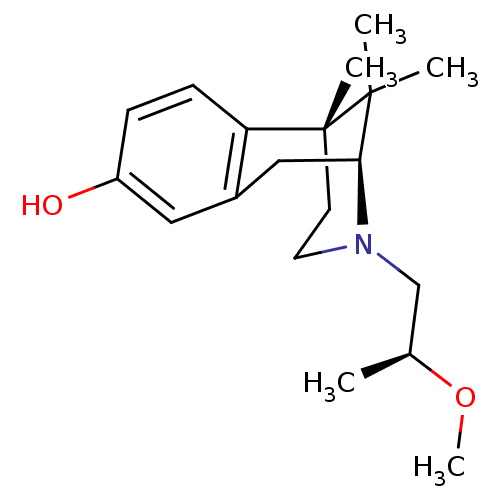 ((2S,6R)-3-((S)-2-Methoxy-propyl)-6,11,11-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059777 ((2R,6S)-3-((R)-2-Methoxy-propyl)-6,11,11-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059782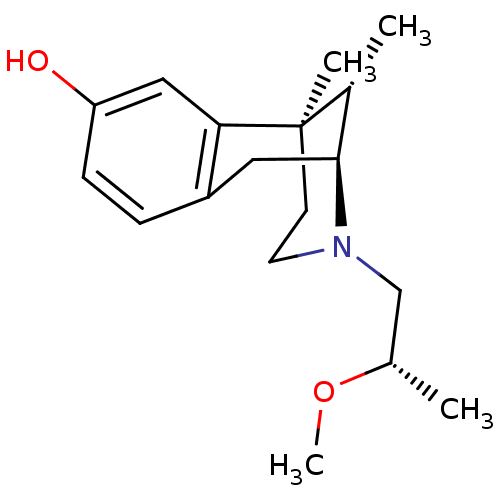 ((2S,6R,11S)-3-((S)-2-Methoxy-propyl)-6,11-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059759 ((2S,6R)-3-((S)-2-Methoxy-propyl)-6,11,11-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059770 ((2R,6R)-3-((R)-2-Methoxy-propyl)-6,11,11-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059776 ((2S,6R)-3-((R)-2-Methoxy-propyl)-6,11,11-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059774 ((2S,6R,11S)-3-((R)-2-Methoxy-propyl)-6,11-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059773 ((2S,6R,11R)-3-((R)-2-Methoxy-propyl)-6,11-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059763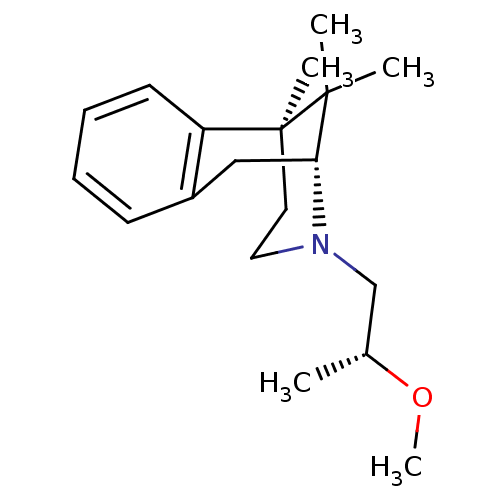 ((2R,6S)-3-((R)-2-Methoxy-propyl)-6,11,11-trimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50059768 ((2S,6R,11R)-3-((S)-2-Methoxy-propyl)-6,11-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-dihydromorphine binding to opioid receptor mu 1 in rat brain cortical membranes. | J Med Chem 40: 2922-30 (1997) Article DOI: 10.1021/jm970131j BindingDB Entry DOI: 10.7270/Q2W66JW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50239773 (CHEMBL492244) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST/His6-tagged GSK3beta (M1 to T420 residues) expressed in baculovirus infected sf9 cells using RBER-IRStide as subst... | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50471928 (CHEMBL141487) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of cAMP hydrolysis by inhibiting phosphodiesterases (PDE4), isolated from tumor tissue of human large cell lung tumor xenograft LXFL-529, ... | J Med Chem 41: 4733-43 (1998) Article DOI: 10.1021/jm981021v BindingDB Entry DOI: 10.7270/Q2SN0CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50239780 (CHEMBL4065365) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of full length human C-terminal His6-tagged CDK2/N-terminal GST-tagged CyclinE expressed in baculovirus infected sf21 cells after 10 mins ... | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50471936 (CHEMBL141890) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of cAMP hydrolysis by inhibiting phosphodiesterases (PDE4), isolated from tumor tissue of human large cell lung tumor xenograft LXFL-529, ... | J Med Chem 41: 4733-43 (1998) Article DOI: 10.1021/jm981021v BindingDB Entry DOI: 10.7270/Q2SN0CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50239778 (CHEMBL1830064) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of compound against thymidylate synthetase | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50471931 (CHEMBL341615) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of cAMP hydrolysis by inhibiting phosphodiesterases (PDE4), isolated from tumor tissue of human large cell lung tumor xenograft LXFL-529, ... | J Med Chem 41: 4733-43 (1998) Article DOI: 10.1021/jm981021v BindingDB Entry DOI: 10.7270/Q2SN0CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50005337 (CHEMBL1276317) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of compound against Guanine-7-methyl transferase | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50471939 (CHEMBL343232) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of cAMP hydrolysis by inhibiting phosphodiesterases (PDE4), isolated from tumor tissue of human large cell lung tumor xenograft LXFL-529, ... | J Med Chem 41: 4733-43 (1998) Article DOI: 10.1021/jm981021v BindingDB Entry DOI: 10.7270/Q2SN0CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50239778 (CHEMBL1830064) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of recombinant GSK3beta (unknown origin) after 10 mins in presence of [gamma32P]ATP by beta counting method | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50239776 (CHEMBL4085289) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Displacement of [125 I] CCK-8 from Cholecystokinin type B receptor of guinea pig cerebral cortex | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50239776 (CHEMBL4085289) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of recombinant GSK3beta (unknown origin) after 10 mins in presence of [gamma32P]ATP by beta counting method | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50321062 (5-{N-[4-(4-Methylpiperazino)-phenyl]-aminocarbonyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of GSK3beta assessed as inhibition of [33Pi] incorporation after 80 mins by microplate scintillation counting | Bioorg Med Chem 18: 4509-15 (2010) Article DOI: 10.1016/j.bmc.2010.04.066 BindingDB Entry DOI: 10.7270/Q2445MN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50005337 (CHEMBL1276317) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of recombinant GSK3beta (unknown origin) after 10 mins in presence of [gamma32P]ATP by beta counting method | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50239778 (CHEMBL1830064) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal His6-tagged CDK6/N-terminal GST-tagged CyclinD3 expressed in sf21 cells after 10 mins in presence of [gamm... | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50239774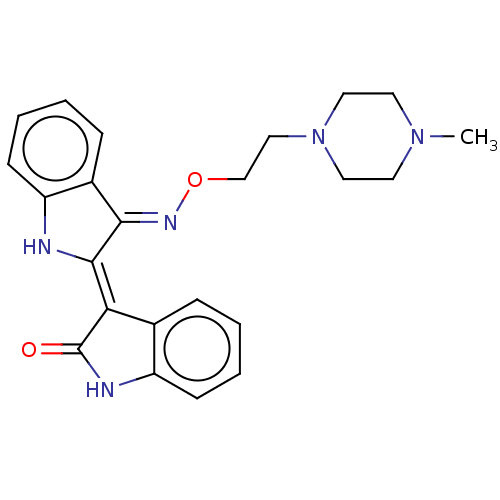 (CHEMBL4084775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of IGF1R (unknown origin) by ADP-Glo assay | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50005337 (CHEMBL1276317) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of full length human C-terminal His6-tagged CDK2/N-terminal GST-tagged CyclinE expressed in baculovirus infected sf21 cells after 10 mins ... | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50239778 (CHEMBL1830064) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of full length human C-terminal His6-tagged CDK2/N-terminal GST-tagged CyclinA expressed in baculovirus infected sf21 cells after 10 mins ... | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50239779 (CHEMBL1802727) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of full length human C-terminal His6-tagged CDK2/N-terminal GST-tagged CyclinE expressed in baculovirus infected sf21 cells after 10 mins ... | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50239777 (CHEMBL4100748) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of recombinant GSK3beta (unknown origin) after 10 mins in presence of [gamma32P]ATP by beta counting method | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50471930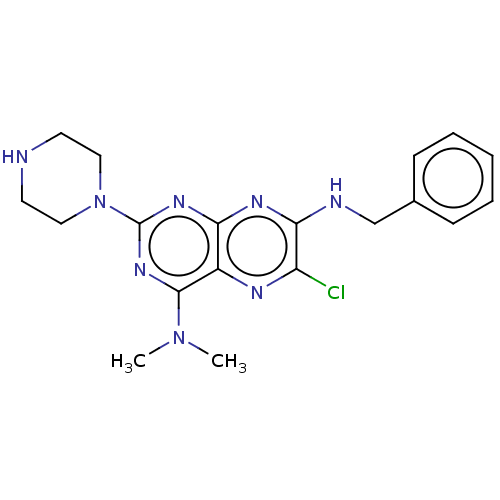 (CHEMBL142504) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of cAMP hydrolysis by inhibiting phosphodiesterases (PDE4), isolated from tumor tissue of human large cell lung tumor xenograft LXFL-529, ... | J Med Chem 41: 4733-43 (1998) Article DOI: 10.1021/jm981021v BindingDB Entry DOI: 10.7270/Q2SN0CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM50239773 (CHEMBL492244) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Displacement of [125 I] CCK-8 from Cholecystokinin type B receptor of guinea pig cerebral cortex | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50471935 (CHEMBL141380) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of cAMP hydrolysis by inhibiting phosphodiesterases (PDE4), isolated from tumor tissue of human large cell lung tumor xenograft LXFL-529, ... | J Med Chem 41: 4733-43 (1998) Article DOI: 10.1021/jm981021v BindingDB Entry DOI: 10.7270/Q2SN0CQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6/G1/S-specific cyclin-D3 (Homo sapiens (Human)) | BDBM50239776 (CHEMBL4085289) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal His6-tagged CDK6/N-terminal GST-tagged CyclinD3 expressed in sf21 cells after 10 mins in presence of [gamm... | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50239773 (CHEMBL492244) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged CDK5 (M1 to P292 residues)/p35NCK (M1 to R307 residues) expressed in baculovirus infected sf9 c... | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50005337 (CHEMBL1276317) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of full length human C-terminal His6-tagged CDK2/N-terminal GST-tagged CyclinA expressed in baculovirus infected sf21 cells after 10 mins ... | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM7392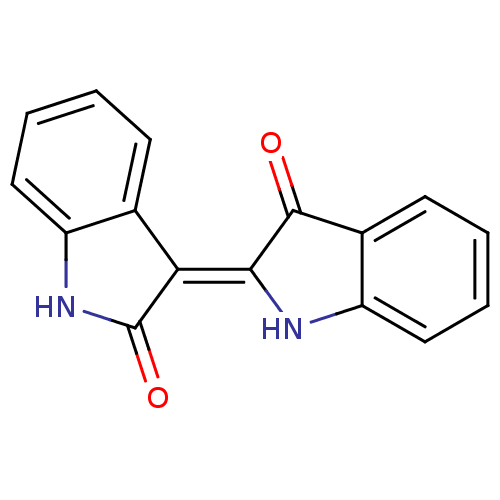 (2-[(3Z)-2-oxo-2,3-dihydro-1H-indol-3-ylidene]-2,3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kaiserslautern Curated by ChEMBL | Assay Description Inhibition of recombinant GSK3beta (unknown origin) after 10 mins in presence of [gamma32P]ATP by beta counting method | J Med Chem 60: 4949-4962 (2017) Article DOI: 10.1021/acs.jmedchem.7b00324 BindingDB Entry DOI: 10.7270/Q2HM5BMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 85 total ) | Next | Last >> |