

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50508208 (CHEMBL4457209) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... | Bioorg Med Chem Lett 29: 441-448 (2019) Article DOI: 10.1016/j.bmcl.2018.12.024 BindingDB Entry DOI: 10.7270/Q2P55RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50508174 (CHEMBL4464625) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... | Bioorg Med Chem Lett 29: 441-448 (2019) Article DOI: 10.1016/j.bmcl.2018.12.024 BindingDB Entry DOI: 10.7270/Q2P55RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289984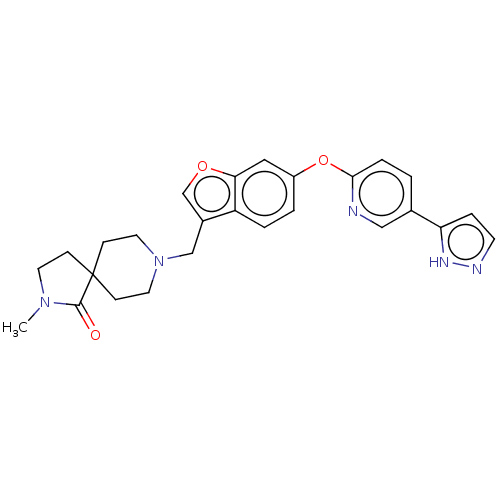 (2-Methyl-8-{6-[5-(2H-pyrazol-3- yl)-pyridin-2-ylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50508174 (CHEMBL4464625) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at CCR1 in human THP1 cells assessed as inhibition of chemotaxis after 30 mins by Celltiter-glo reagent based luminescence assay | Bioorg Med Chem Lett 29: 441-448 (2019) Article DOI: 10.1016/j.bmcl.2018.12.024 BindingDB Entry DOI: 10.7270/Q2P55RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50508182 (CHEMBL4475581) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at recombinant CCR1 (unknown origin) expressed in non-adherent cells co-expressing Galpha16 assessed as inhibition of MIP-1 alpha... | Bioorg Med Chem Lett 29: 441-448 (2019) Article DOI: 10.1016/j.bmcl.2018.12.024 BindingDB Entry DOI: 10.7270/Q2P55RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289920 (2-Methoxy-1-(8-{6-[5-(2H-pyrazol-3-yl)-pyridin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289869 (N-((R)-1-{6-[5-(2H-Pyrazol-3-yl)- pyridin-2-yloxy]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289983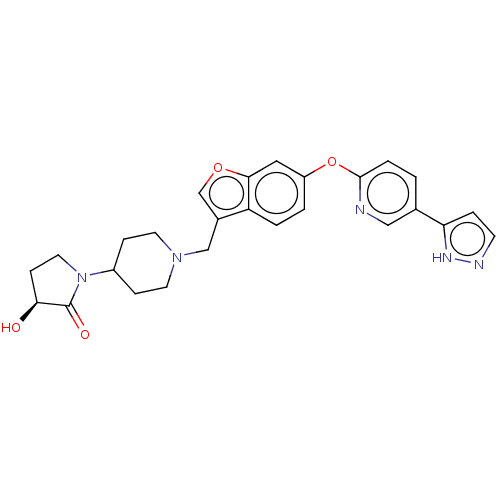 ((S)-3-Hydroxy-1-(1-{6-[5-(2H- pyrazol-3-yl)-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289980 (N-(1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289864 (2-Hydroxy-1-(4-{6-[5-(2H-pyrazol-3- yl)-pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289987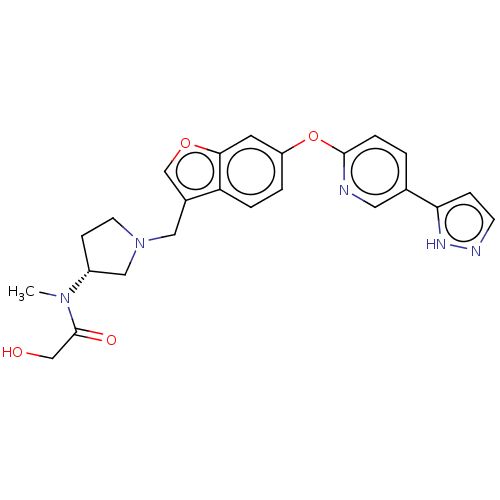 (2-Hydroxy-N-methyl-N-((R)-1-{6- [5-(2H-pyrazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289989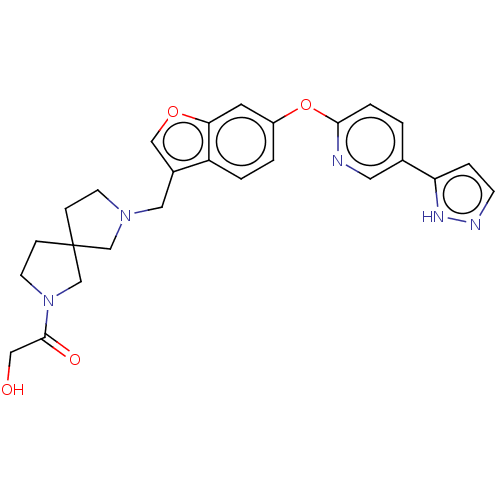 (2-Hydroxy-1-(7-{6-[5-(2H-pyrazol- 3-yl)-pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289917 ((S)-2-Hydroxy-1-(8-{6-[5-(2H-pyrazol-3-yl)-pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289897 (1-(4-{6-[5-(2H-Pyrazol-3-yl)-pyrimidin- 2-yloxy]-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289858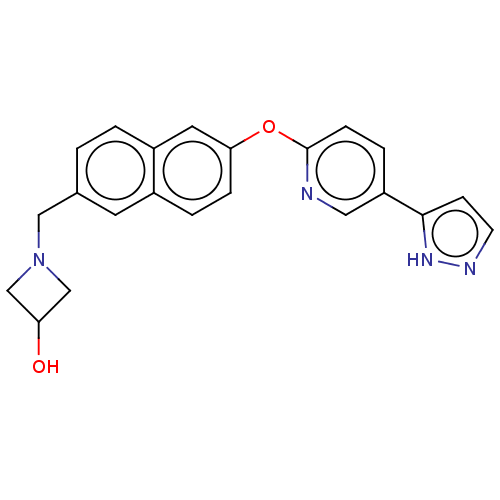 (1-{6-[5-(2H-Pyrazol-3-yl)-pyridin-2-yloxy]-naphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289918 (2-Hydroxy-2-methyl-1-(8-{6-[5-(2H- pyrazol-3-yl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289859 (1-{6-[5-(2H-Pyrazol-3-yl)-pyridin-2- yloxy]-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289932 (1-[1-(2-{6-[5-(2H-Pyrazol-3-yl)- pyridin-2-yloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM290023 (2-Methyl-3-oxo-3-(4-{6-[5-(2H-pyrazol- 3-yl)-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289867 (2-{6-[5-(2H-Pyrazol-3-yl)-pyridin-2-yloxy]-naphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289861 ((1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289976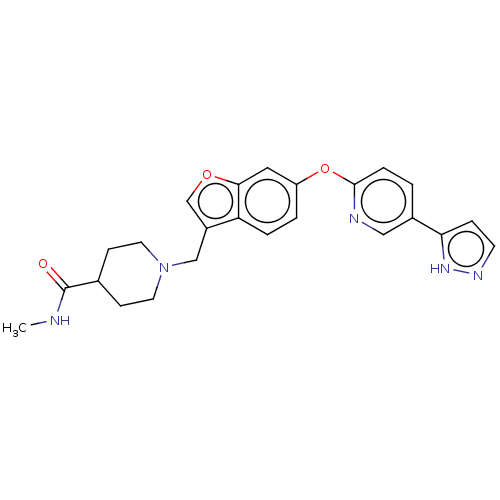 (1-{6-[5-(2H-Pyrazol-3-yl)-pyridin-2- yloxy]-benzof...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human foreskin fibroblasts assessed as inhibition of IL-1-induced IL-6 production by trans-repression ... | Bioorg Med Chem Lett 24: 1934-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.005 BindingDB Entry DOI: 10.7270/Q2251KPB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289866 (2-Hydroxy-N-(1-{6-[5-(2H-pyrazol-3- yl)-pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289860 (N-(1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer-Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in HFF assessed as inhibition of IL-1-induced IL-6 production after 18 to 24 hrs by ELISA | J Med Chem 53: 6681-98 (2010) Article DOI: 10.1021/jm100751q BindingDB Entry DOI: 10.7270/Q2571D0X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289916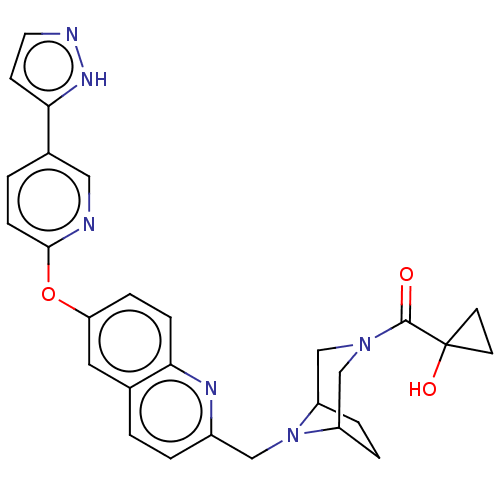 ((1-Hydroxy-cyclopropyl)-(8-{6-[5- (2H-pyrazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289868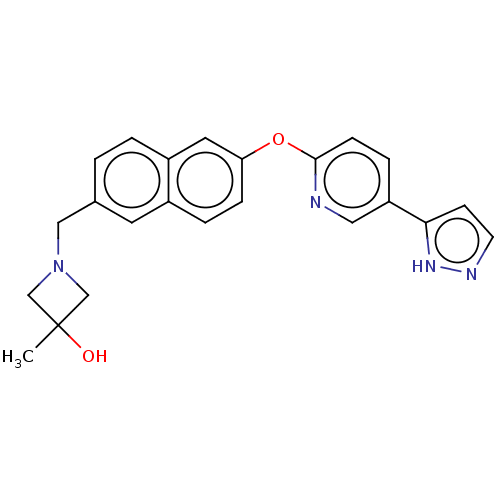 (3-Methyl-1-{6-[5-(2H-pyrazol-3- yl)-pyridin-2-ylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289919 ((R)-2-Hydroxy-1-(8-{6-[5-(2H-pyrazol-3-yl)-pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289988 (2-Hydroxy-N-methyl-N-((S)-1-{6- [5-(2H-pyrazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289977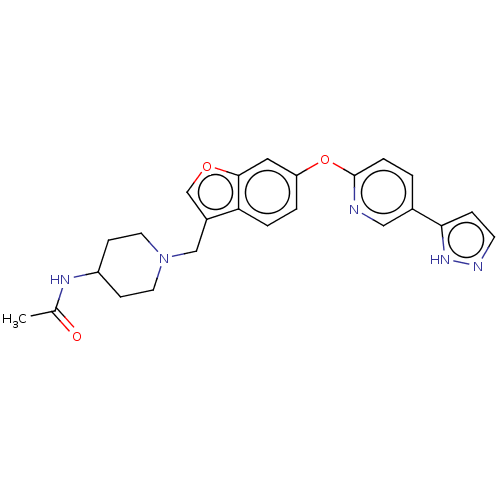 (N-(1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM290002 (2-Methoxy-2-methyl-1-(4-{6- [5-(2H-pyrazol-3-yl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289863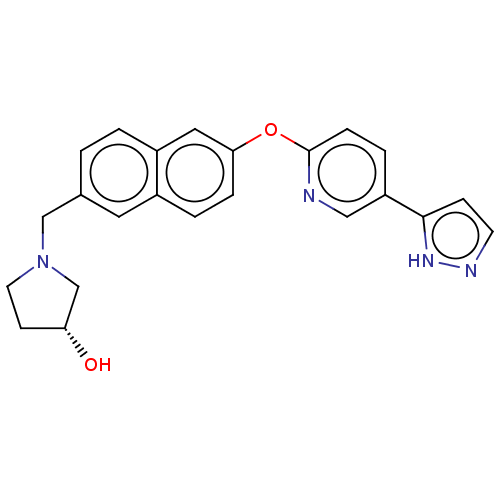 ((R)-1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM290014 ((R)-2-Methoxy-1-(4-{6-[5-(2H-pyrazol- 3-yl)-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289970 (1-(4-{6-[5-(2H-Pyrazol-3-yl)-pyridin-2-yloxy]-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289968 (1-(4-{6-[4-(2H-Pyrazol-3-yl)- phenoxy]-imidazo[1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289862 ((S)-1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM303361 (US10138222, Example 76) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description In a 384-well plate, test compound diluted in assay buffer (1% DMSO final) is mixed with 8His-RIPK2 FL enzyme (final concentration of 8 nM). After 15... | US Patent US10138222 (2018) BindingDB Entry DOI: 10.7270/Q2RV0QS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289878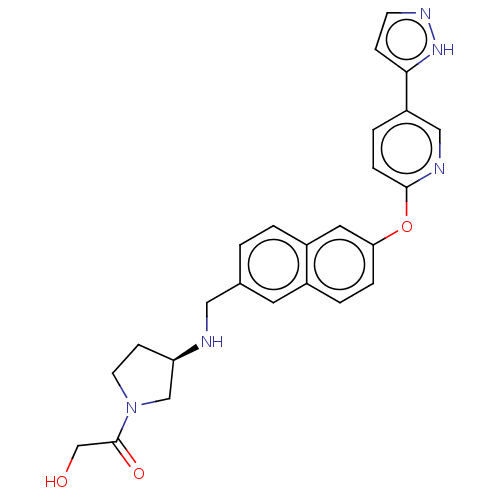 (2-Hydroxy-1-[(R)-3-(methyl-{6-[5-(2H-pyrazol-3-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289881 (((R)-1-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289913 ((S)-2-Methoxy-1-(8-{6-[5-(2H-pyrazol- 3-yl)-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289975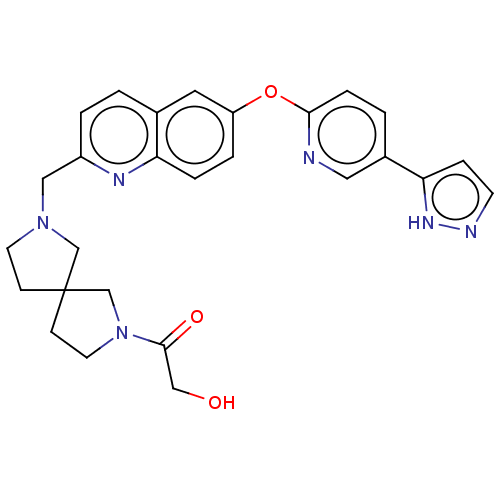 (2-Hydroxy-1-(7-{6-[5-(2H-pyrazol- 3-yl)-pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289956 (2-Methoxy-1-(4-{6-[4-(2H-pyrazol- 3-yl)-phenoxy]-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289923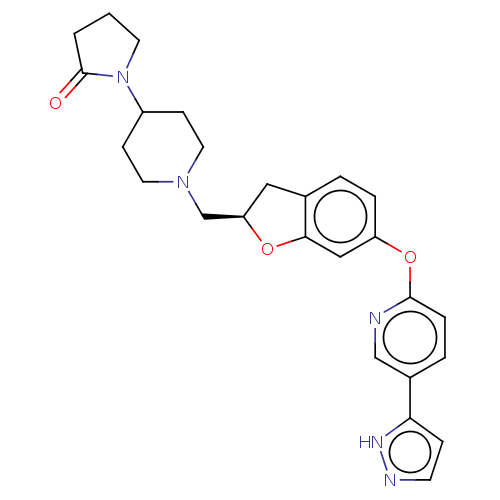 (1-(1-{(R)-6-[5-(2H-Pyrazol-3-yl)- pyridin-2-yloxy]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289880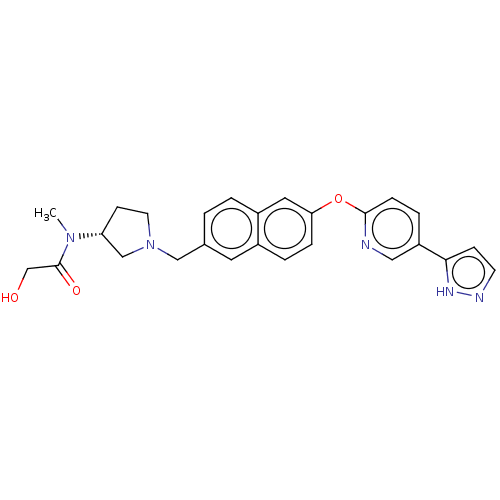 (2-Hydroxy-N-methyl-N-((R)-1-{6-[5- (2H-pyrazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289992 ((S)-2-Methoxy-1-(4-{6-[5-(2H-pyrazol- 3-yl)-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289985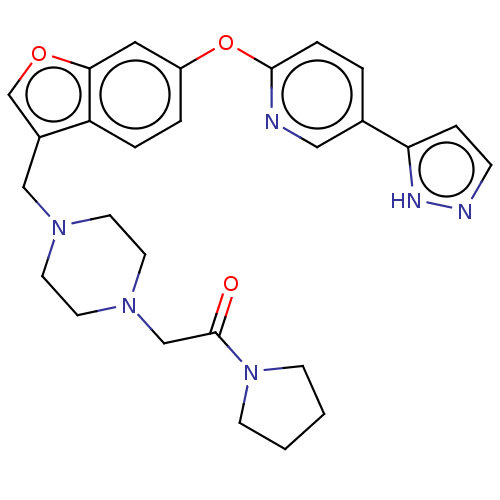 (2-(4-{6-[5-(2H-Pyrazol-3-yl)-pyridin- 2-yloxy]-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289910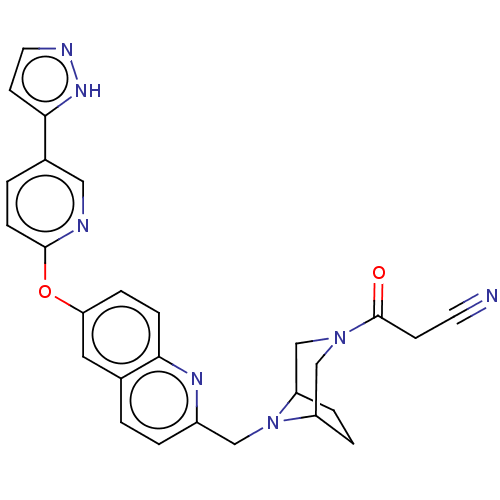 (3-Oxo-3-(8-{6-[5-(2H-pyrazol-3-yl)-pyridin-2-yloxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289981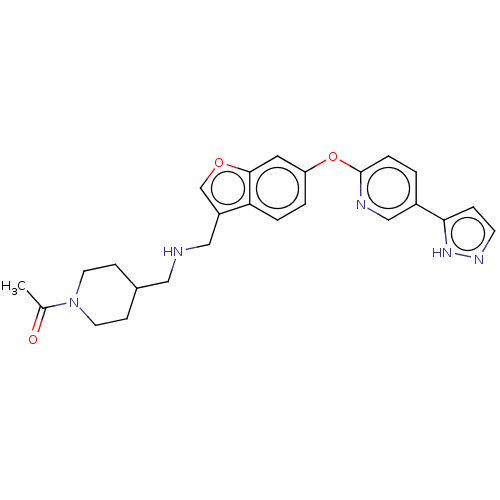 (1-{4-[({6-[5-(2H-Pyrazol-3-yl)- pyridin-2-yloxy]-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM289874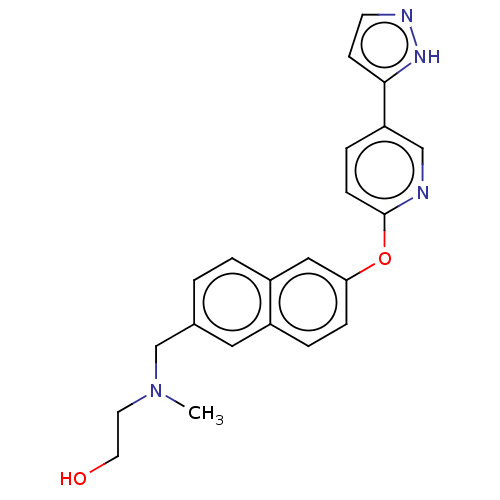 (2-(Methyl-{6-[5-(2H-pyrazol-3-yl)- pyridin-2-yloxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description The compounds of the invention are assessed for the ability to interact with human LTA4 hydrolase in an enzymatic assay that measures the ability of ... | US Patent US9573957 (2017) BindingDB Entry DOI: 10.7270/Q27S7QTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 975 total ) | Next | Last >> |