 Found 281 hits with Last Name = 'takano' and Initial = 'h'
Found 281 hits with Last Name = 'takano' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082445
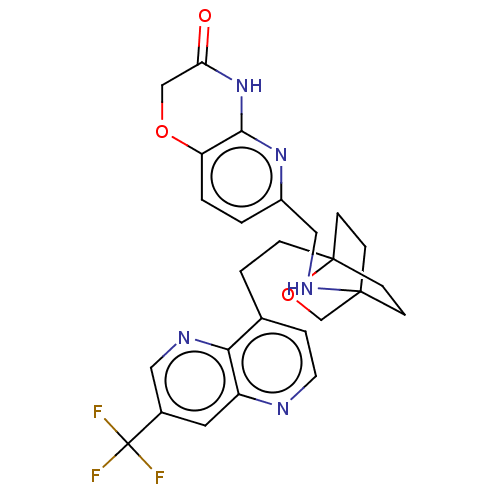
(CHEMBL3422978)Show SMILES FC(F)(F)c1cnc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2c1 |(-5.09,-.93,;-4.02,-1.54,;-4.02,-2.78,;-5.09,-2.16,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C26H26F3N5O3/c27-26(28,29)17-11-19-22(31-12-17)16(4-10-30-19)3-5-25-8-6-24(7-9-25,15-37-25)32-13-18-1-2-20-23(33-18)34-21(35)14-36-20/h1-2,4,10-12,32H,3,5-9,13-15H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082380
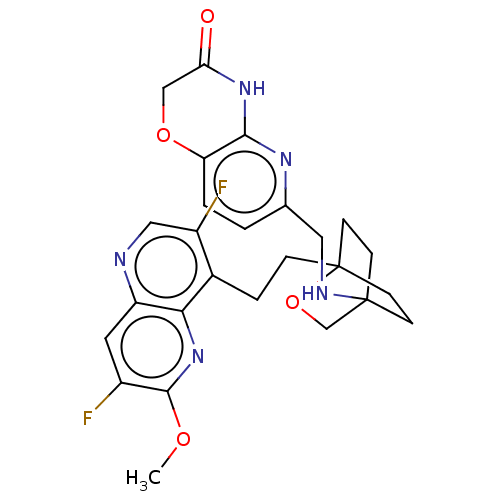
(CHEMBL3422952)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c(F)cnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C26H27F2N5O4/c1-35-24-17(27)10-19-22(33-24)16(18(28)12-29-19)4-5-26-8-6-25(7-9-26,14-37-26)30-11-15-2-3-20-23(31-15)32-21(34)13-36-20/h2-3,10,12,30H,4-9,11,13-14H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082429

(CHEMBL3422970)Show SMILES Cc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C#N |(-3.75,1.39,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-2.16,)| Show InChI InChI=1S/C27H28N6O3/c1-17-19(13-28)12-21-24(31-17)18(5-11-29-21)4-6-27-9-7-26(8-10-27,16-36-27)30-14-20-2-3-22-25(32-20)33-23(34)15-35-22/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082385
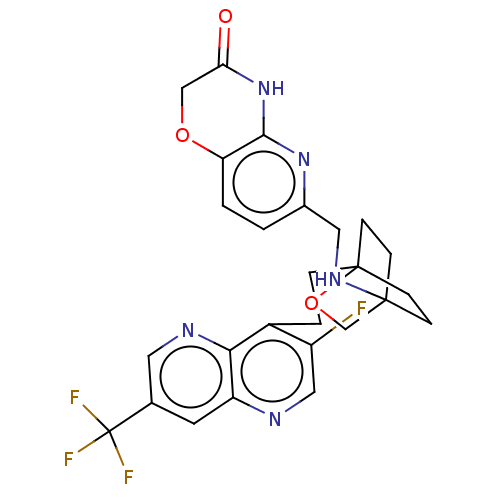
(CHEMBL3422959)Show SMILES Fc1cnc2cc(cnc2c1CCC12CCC(CC1)(CO2)NCc1ccc2OCC(=O)Nc2n1)C(F)(F)F Show InChI InChI=1S/C26H25F4N5O3/c27-18-12-31-19-9-15(26(28,29)30)10-32-22(19)17(18)3-4-25-7-5-24(6-8-25,14-38-25)33-11-16-1-2-20-23(34-16)35-21(36)13-37-20/h1-2,9-10,12,33H,3-8,11,13-14H2,(H,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082440

(CHEMBL3422977)Show SMILES FC(F)(F)c1ccc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-5.09,.93,;-4.02,1.54,;-4.02,2.78,;-5.09,2.16,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C26H26F3N5O3/c27-26(28,29)20-4-2-18-22(33-20)16(6-12-30-18)5-7-25-10-8-24(9-11-25,15-37-25)31-13-17-1-3-19-23(32-17)34-21(35)14-36-19/h1-4,6,12,31H,5,7-11,13-15H2,(H,32,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082435
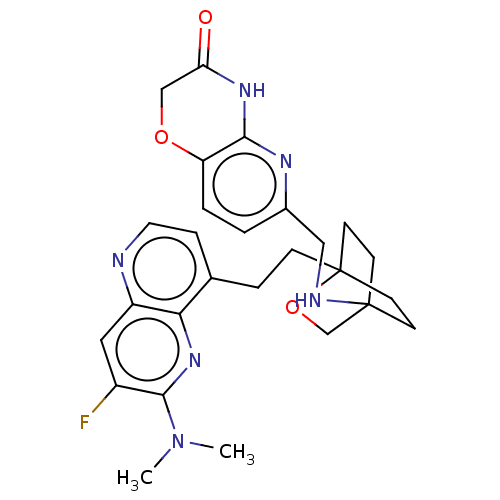
(CHEMBL3422976)Show SMILES CN(C)c1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-5.09,.93,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C27H31FN6O3/c1-34(2)25-19(28)13-20-23(33-25)17(6-12-29-20)5-7-27-10-8-26(9-11-27,16-37-27)30-14-18-3-4-21-24(31-18)32-22(35)15-36-21/h3-4,6,12-13,30H,5,7-11,14-16H2,1-2H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285498
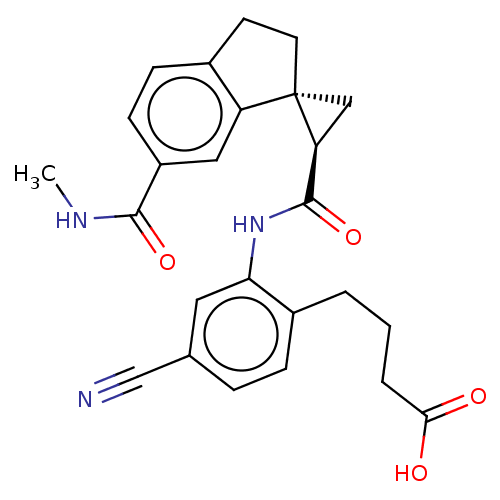
(4-[4-Cyano-2-({[(1R,2R)-6′-(methylcarbamoyl)...)Show SMILES CNC(=O)c1ccc2CC[C@]3(C[C@H]3C(=O)Nc3cc(ccc3CCCC(O)=O)C#N)c2c1 |r| Show InChI InChI=1S/C25H25N3O4/c1-27-23(31)18-8-7-16-9-10-25(19(16)12-18)13-20(25)24(32)28-21-11-15(14-26)5-6-17(21)3-2-4-22(29)30/h5-8,11-12,20H,2-4,9-10,13H2,1H3,(H,27,31)(H,28,32)(H,29,30)/t20-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082382
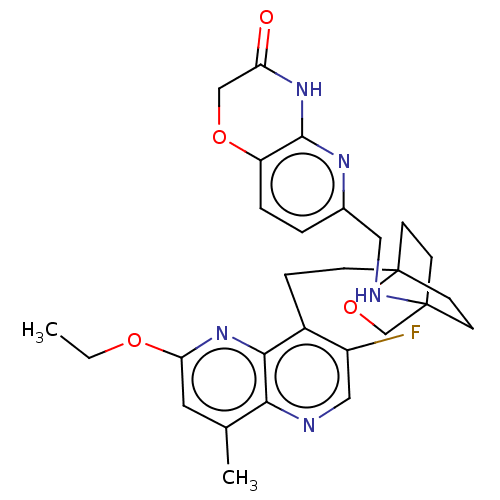
(CHEMBL3422954)Show SMILES CCOc1cc(C)c2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-5.09,3.71,;-4.02,3.09,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-2.77,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C28H32FN5O4/c1-3-36-23-12-17(2)24-25(34-23)19(20(29)14-30-24)6-7-28-10-8-27(9-11-28,16-38-28)31-13-18-4-5-21-26(32-18)33-22(35)15-37-21/h4-5,12,14,31H,3,6-11,13,15-16H2,1-2H3,(H,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285464
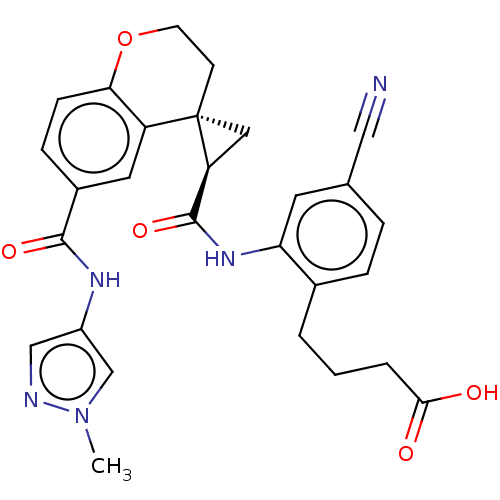
(4-{4-Cyano-2-[({2′R, 4S)-6-[(1-methyl 1H-pyr...)Show SMILES Cn1cc(NC(=O)c2ccc3OCC[C@]4(C[C@H]4C(=O)Nc4cc(ccc4CCCC(O)=O)C#N)c3c2)cn1 |r| Show InChI InChI=1S/C28H27N5O5/c1-33-16-20(15-30-33)31-26(36)19-7-8-24-21(12-19)28(9-10-38-24)13-22(28)27(37)32-23-11-17(14-29)5-6-18(23)3-2-4-25(34)35/h5-8,11-12,15-16,22H,2-4,9-10,13H2,1H3,(H,31,36)(H,32,37)(H,34,35)/t22-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082390
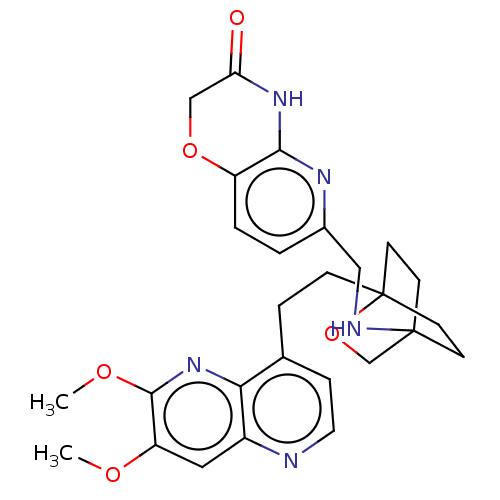
(CHEMBL3422964)Show SMILES COc1cc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2nc1OC |(-4.02,-2.78,;-4.02,-1.54,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,;-2.69,.77,;-4.02,1.54,;-4.02,2.78,)| Show InChI InChI=1S/C27H31N5O5/c1-34-21-13-19-23(32-25(21)35-2)17(6-12-28-19)5-7-27-10-8-26(9-11-27,16-37-27)29-14-18-3-4-20-24(30-18)31-22(33)15-36-20/h3-4,6,12-13,29H,5,7-11,14-16H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082394

(CHEMBL3422966)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C#N |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-2.16,)| Show InChI InChI=1S/C27H28N6O4/c1-35-25-18(13-28)12-20-23(33-25)17(5-11-29-20)4-6-27-9-7-26(8-10-27,16-37-27)30-14-19-2-3-21-24(31-19)32-22(34)15-36-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285502

(US10077247, Example 20-2)Show SMILES COCCNC(=O)c1cc2c(OCC[C@]22C[C@H]2C(=O)Nc2cc(ccc2CCCC(O)=O)C#N)cc1F |r| Show InChI InChI=1S/C27H28FN3O6/c1-36-10-8-30-25(34)18-12-19-23(13-21(18)28)37-9-7-27(19)14-20(27)26(35)31-22-11-16(15-29)5-6-17(22)3-2-4-24(32)33/h5-6,11-13,20H,2-4,7-10,14H2,1H3,(H,30,34)(H,31,35)(H,32,33)/t20-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082388
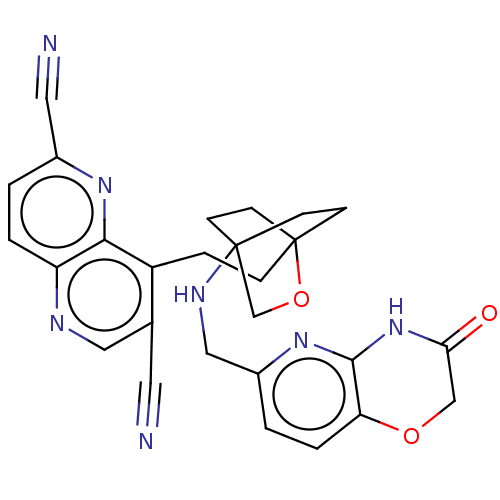
(CHEMBL3422962)Show SMILES O=C1COc2ccc(CNC34CCC(CCc5c(cnc6ccc(nc56)C#N)C#N)(CC3)OC4)nc2N1 |(10.96,15.37,;9.76,15.09,;8.71,16.22,;7.21,15.87,;6.76,14.39,;5.26,14.04,;4.8,12.56,;5.86,11.44,;5.41,9.96,;3.9,9.61,;3.46,8.16,;4.56,7.06,;4.17,5.57,;2.68,5.4,;2.67,3.85,;1.34,3.08,;1.33,1.54,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;-4.02,1.54,;-5.09,2.16,;4,1.54,;5.07,2.16,;2.27,6.67,;3.81,6.67,;1.58,6.27,;1.98,7.74,;7.37,11.8,;7.81,13.27,;9.31,13.62,)| Show InChI InChI=1S/C27H25N7O3/c28-11-17-13-30-21-3-1-18(12-29)32-24(21)20(17)5-6-27-9-7-26(8-10-27,16-37-27)31-14-19-2-4-22-25(33-19)34-23(35)15-36-22/h1-4,13,31H,5-10,14-16H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082383

(CHEMBL3422957)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c(F)cnc2cc1C(F)(F)F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-.93,;-4.02,-2.78,;-5.09,-2.16,)| Show InChI InChI=1S/C27H27F4N5O4/c1-38-24-17(27(29,30)31)10-19-22(36-24)16(18(28)12-32-19)4-5-26-8-6-25(7-9-26,14-40-26)33-11-15-2-3-20-23(34-15)35-21(37)13-39-20/h2-3,10,12,33H,4-9,11,13-14H2,1H3,(H,34,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082427
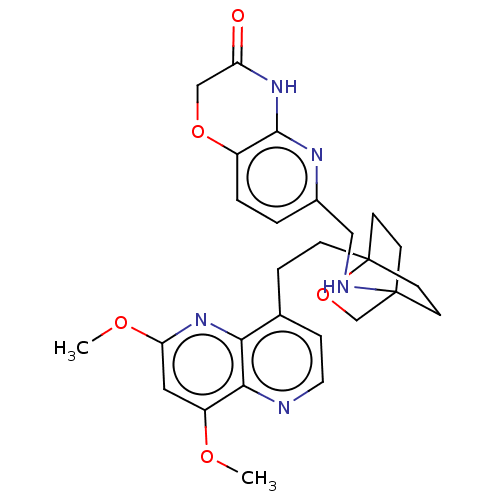
(CHEMBL3422968)Show SMILES COc1cc(OC)c2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-3.08,;-2.39,-3.71,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H31N5O5/c1-34-20-13-22(35-2)32-23-17(6-12-28-24(20)23)5-7-27-10-8-26(9-11-27,16-37-27)29-14-18-3-4-19-25(30-18)31-21(33)15-36-19/h3-4,6,12-13,29H,5,7-11,14-16H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082430
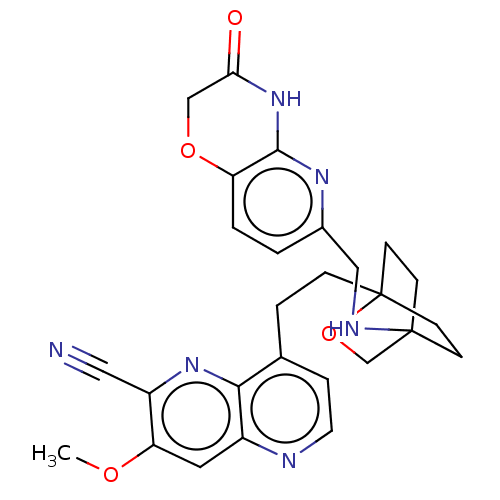
(CHEMBL3422971)Show SMILES COc1cc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2nc1C#N |(-4.02,-2.78,;-4.02,-1.54,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,;-2.69,.77,;-4.02,1.54,;-5.09,2.16,)| Show InChI InChI=1S/C27H28N6O4/c1-35-22-12-19-24(32-20(22)13-28)17(5-11-29-19)4-6-27-9-7-26(8-10-27,16-37-27)30-14-18-2-3-21-25(31-18)33-23(34)15-36-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082432
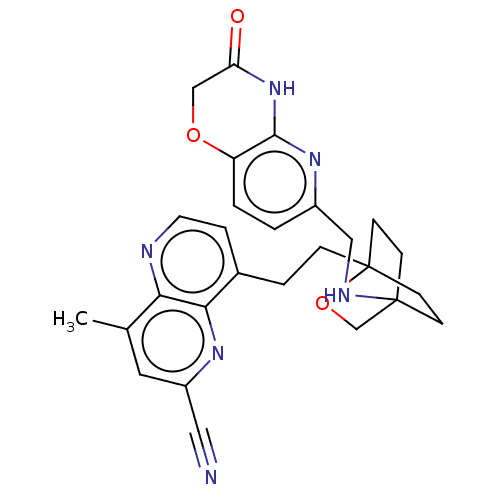
(CHEMBL3422973)Show SMILES Cc1cc(nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc12)C#N |(-1.33,-2.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-4.02,1.54,;-5.09,2.16,)| Show InChI InChI=1S/C27H28N6O3/c1-17-12-20(13-28)31-24-18(5-11-29-23(17)24)4-6-27-9-7-26(8-10-27,16-36-27)30-14-19-2-3-21-25(32-19)33-22(34)15-35-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082520

(CHEMBL3422983)Show SMILES COc1ccc2ncc(C#N)c(CC(O)C34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;4,1.54,;5.07,2.16,;1.33,1.54,;1.34,3.08,;2.67,3.85,;3.74,3.23,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H28N6O5/c1-36-23-5-3-19-24(33-23)18(16(11-28)12-29-19)10-21(34)27-8-6-26(7-9-27,15-38-27)30-13-17-2-4-20-25(31-17)32-22(35)14-37-20/h2-5,12,21,30,34H,6-10,13-15H2,1H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082520

(CHEMBL3422983)Show SMILES COc1ccc2ncc(C#N)c(CC(O)C34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;4,1.54,;5.07,2.16,;1.33,1.54,;1.34,3.08,;2.67,3.85,;3.74,3.23,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H28N6O5/c1-36-23-5-3-19-24(33-23)18(16(11-28)12-29-19)10-21(34)27-8-6-26(7-9-27,15-38-27)30-13-17-2-4-20-25(31-17)32-22(35)14-37-20/h2-5,12,21,30,34H,6-10,13-15H2,1H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082434

(CHEMBL3422975)Show SMILES CN(C)c1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C(F)(F)F |(-4.02,2.78,;-4.02,1.54,;-5.09,.93,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-.93,;-4.02,-2.78,;-5.09,-2.16,)| Show InChI InChI=1S/C28H31F3N6O3/c1-37(2)25-19(28(29,30)31)13-20-23(36-25)17(6-12-32-20)5-7-27-10-8-26(9-11-27,16-40-27)33-14-18-3-4-21-24(34-18)35-22(38)15-39-21/h3-4,6,12-13,33H,5,7-11,14-16H2,1-2H3,(H,34,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285487
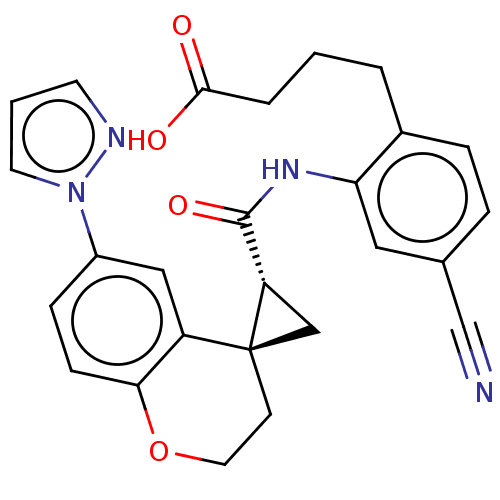
(4-[4-Cyano-2-({[(2′R,4S)-6-(1H-pyrazol-1-yl)...)Show SMILES OC(=O)CCCc1ccc(cc1NC(=O)[C@@H]1C[C@]11CCOc2ccc(cc12)-n1cccn1)C#N |r| Show InChI InChI=1S/C26H24N4O4/c27-16-17-5-6-18(3-1-4-24(31)32)22(13-17)29-25(33)21-15-26(21)9-12-34-23-8-7-19(14-20(23)26)30-11-2-10-28-30/h2,5-8,10-11,13-14,21H,1,3-4,9,12,15H2,(H,29,33)(H,31,32)/t21-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082391
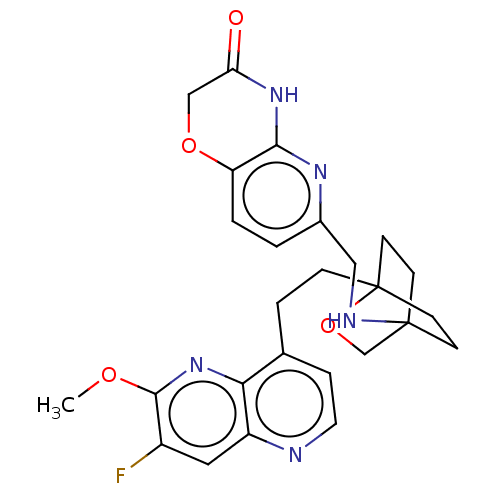
(CHEMBL3422965)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C26H28FN5O4/c1-34-24-18(27)12-19-22(32-24)16(5-11-28-19)4-6-26-9-7-25(8-10-26,15-36-26)29-13-17-2-3-20-23(30-17)31-21(33)14-35-20/h2-3,5,11-12,29H,4,6-10,13-15H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285036
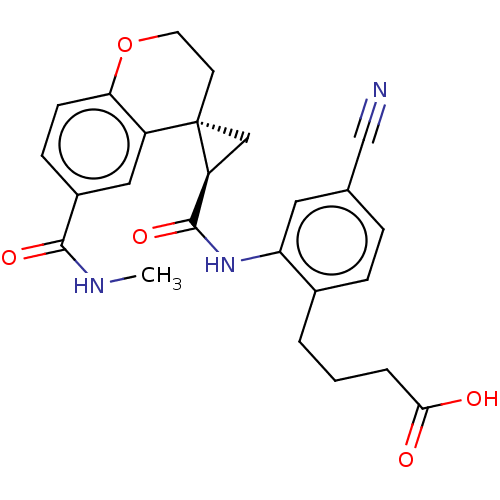
(US10077247, Example 1)Show SMILES CNC(=O)c1ccc2OCC[C@]3(C[C@H]3C(=O)Nc3cc(ccc3CCCC(O)=O)C#N)c2c1 |r| Show InChI InChI=1S/C25H25N3O5/c1-27-23(31)17-7-8-21-18(12-17)25(9-10-33-21)13-19(25)24(32)28-20-11-15(14-26)5-6-16(20)3-2-4-22(29)30/h5-8,11-12,19H,2-4,9-10,13H2,1H3,(H,27,31)(H,28,32)(H,29,30)/t19-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285470

(4-[4-Cyano-2-({[(2′R,4S)-6-(3-pyridazinylcar...)Show SMILES OC(=O)CCCc1ccc(cc1NC(=O)[C@@H]1C[C@]11CCOc2ccc(cc12)C(=O)Nc1cccnn1)C#N |r| Show InChI InChI=1S/C28H25N5O5/c29-16-17-6-7-18(3-1-5-25(34)35)22(13-17)31-27(37)21-15-28(21)10-12-38-23-9-8-19(14-20(23)28)26(36)32-24-4-2-11-30-33-24/h2,4,6-9,11,13-14,21H,1,3,5,10,12,15H2,(H,31,37)(H,34,35)(H,32,33,36)/t21-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285480
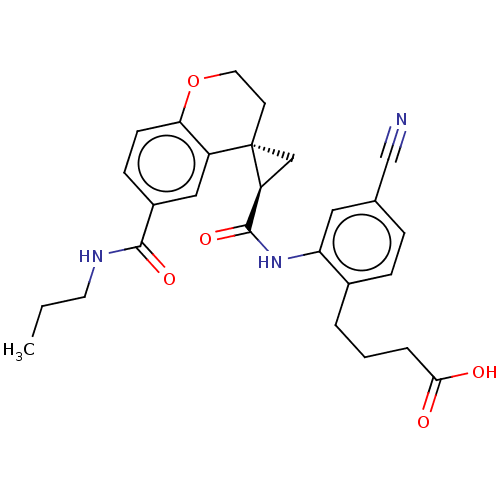
(4-[4-Cyano-2-({[(2′R,4S)-6-(propylcarbamoyl)...)Show SMILES CCCNC(=O)c1ccc2OCC[C@]3(C[C@H]3C(=O)Nc3cc(ccc3CCCC(O)=O)C#N)c2c1 |r| Show InChI InChI=1S/C27H29N3O5/c1-2-11-29-25(33)19-8-9-23-20(14-19)27(10-12-35-23)15-21(27)26(34)30-22-13-17(16-28)6-7-18(22)4-3-5-24(31)32/h6-9,13-14,21H,2-5,10-12,15H2,1H3,(H,29,33)(H,30,34)(H,31,32)/t21-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285483
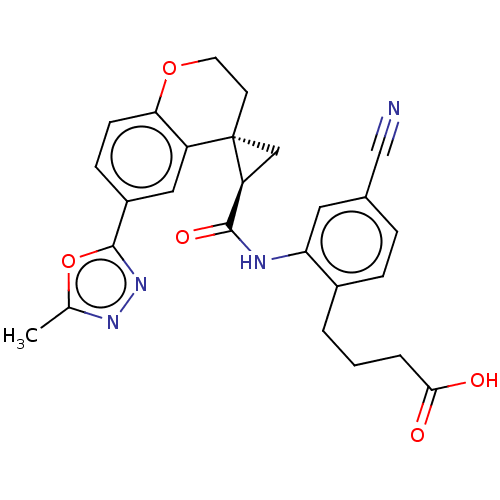
(4-[4-Cyano-2-({[(2′R,4S)-6-(5-methyl-1,3,4-o...)Show SMILES Cc1nnc(o1)-c1ccc2OCC[C@]3(C[C@H]3C(=O)Nc3cc(ccc3CCCC(O)=O)C#N)c2c1 |r| Show InChI InChI=1S/C26H24N4O5/c1-15-29-30-25(35-15)18-7-8-22-19(12-18)26(9-10-34-22)13-20(26)24(33)28-21-11-16(14-27)5-6-17(21)3-2-4-23(31)32/h5-8,11-12,20H,2-4,9-10,13H2,1H3,(H,28,33)(H,31,32)/t20-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285500
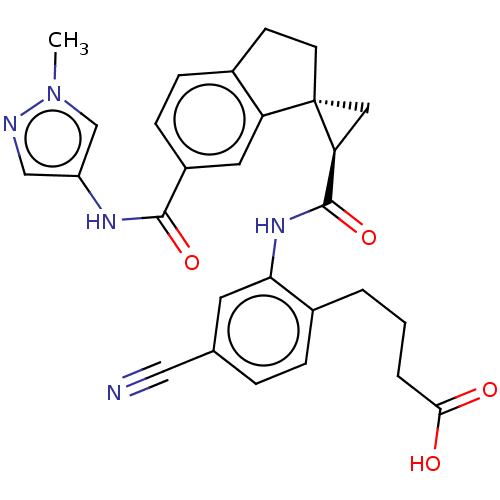
(4-{4-Cyano-2-[({(1R,2R)-6′-[(1-methyl-1H-pyr...)Show SMILES Cn1cc(NC(=O)c2ccc3CC[C@]4(C[C@H]4C(=O)Nc4cc(ccc4CCCC(O)=O)C#N)c3c2)cn1 |r| Show InChI InChI=1S/C28H27N5O4/c1-33-16-21(15-30-33)31-26(36)20-8-7-18-9-10-28(22(18)12-20)13-23(28)27(37)32-24-11-17(14-29)5-6-19(24)3-2-4-25(34)35/h5-8,11-12,15-16,23H,2-4,9-10,13H2,1H3,(H,31,36)(H,32,37)(H,34,35)/t23-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285484

(US10077247, Example 5)Show SMILES Cc1noc(n1)-c1ccc2OCC[C@]3(C[C@H]3C(=O)Nc3cc(ccc3CCCC(O)=O)C#N)c2c1 |r| Show InChI InChI=1S/C26H24N4O5/c1-15-28-25(35-30-15)18-7-8-22-19(12-18)26(9-10-34-22)13-20(26)24(33)29-21-11-16(14-27)5-6-17(21)3-2-4-23(31)32/h5-8,11-12,20H,2-4,9-10,13H2,1H3,(H,29,33)(H,31,32)/t20-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082381

(CHEMBL3422953)Show SMILES COc1cc(C)c2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-2.77,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-11-22(35-2)33-24-18(19(28)13-29-23(16)24)5-6-27-9-7-26(8-10-27,15-37-27)30-12-17-3-4-20-25(31-17)32-21(34)14-36-20/h3-4,11,13,30H,5-10,12,14-15H2,1-2H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285499
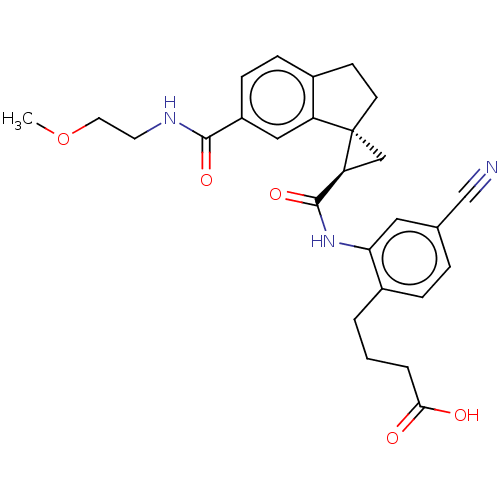
(4-{4-Cyano-2-[({(1R,2R)-6′-[(2-methoxyethyl)...)Show SMILES COCCNC(=O)c1ccc2CC[C@]3(C[C@H]3C(=O)Nc3cc(ccc3CCCC(O)=O)C#N)c2c1 |r| Show InChI InChI=1S/C27H29N3O5/c1-35-12-11-29-25(33)20-8-7-18-9-10-27(21(18)14-20)15-22(27)26(34)30-23-13-17(16-28)5-6-19(23)3-2-4-24(31)32/h5-8,13-14,22H,2-4,9-12,15H2,1H3,(H,29,33)(H,30,34)(H,31,32)/t22-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285482
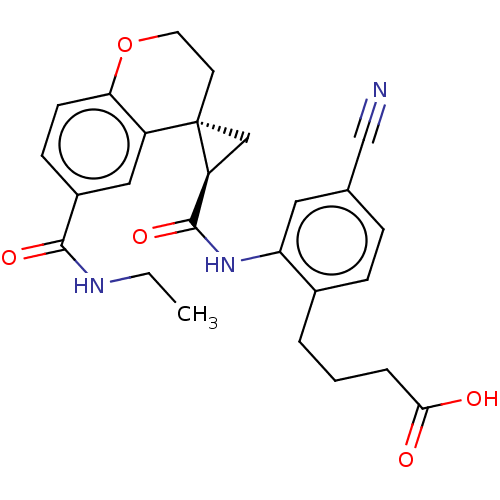
(4-[4-Cyano-2-({[(2′R,4S)-6-(ethylcarbamoyl)-...)Show SMILES CCNC(=O)c1ccc2OCC[C@]3(C[C@H]3C(=O)Nc3cc(ccc3CCCC(O)=O)C#N)c2c1 |r| Show InChI InChI=1S/C26H27N3O5/c1-2-28-24(32)18-8-9-22-19(13-18)26(10-11-34-22)14-20(26)25(33)29-21-12-16(15-27)6-7-17(21)4-3-5-23(30)31/h6-9,12-13,20H,2-5,10-11,14H2,1H3,(H,28,32)(H,29,33)(H,30,31)/t20-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082376

(CHEMBL3422948)Show SMILES Nc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-3.75,1.39,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C25H27FN6O3/c26-17-12-28-18-2-4-20(27)31-22(18)16(17)5-6-25-9-7-24(8-10-25,14-35-25)29-11-15-1-3-19-23(30-15)32-21(33)13-34-19/h1-4,12,29H,5-11,13-14H2,(H2,27,31)(H,30,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285463
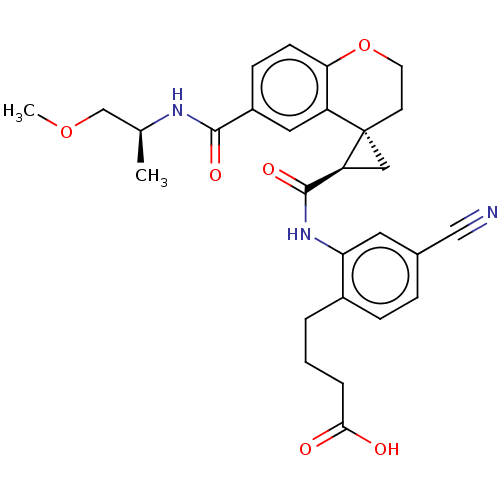
(US10077247, Example 2-4)Show SMILES COC[C@H](C)NC(=O)c1ccc2OCC[C@]3(C[C@H]3C(=O)Nc3cc(ccc3CCCC(O)=O)C#N)c2c1 |r| Show InChI InChI=1S/C28H31N3O6/c1-17(16-36-2)30-26(34)20-8-9-24-21(13-20)28(10-11-37-24)14-22(28)27(35)31-23-12-18(15-29)6-7-19(23)4-3-5-25(32)33/h6-9,12-13,17,22H,3-5,10-11,14,16H2,1-2H3,(H,30,34)(H,31,35)(H,32,33)/t17-,22-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082387

(CHEMBL3422961)Show SMILES Fc1cnc2cc(Cl)cnc2c1CCC12CCC(CC1)(CO2)NCc1ccc2OCC(=O)Nc2n1 |(3.74,1.39,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,)| Show InChI InChI=1S/C25H25ClFN5O3/c26-15-9-19-22(29-10-15)17(18(27)12-28-19)3-4-25-7-5-24(6-8-25,14-35-25)30-11-16-1-2-20-23(31-16)32-21(33)13-34-20/h1-2,9-10,12,30H,3-8,11,13-14H2,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285478

(4-[4-Cyano-2-({[(2′R,4S)-6-(1,2-oxazol-5-ylc...)Show SMILES OC(=O)CCCc1ccc(cc1NC(=O)[C@@H]1C[C@]11CCOc2ccc(cc12)C(=O)Nc1ccno1)C#N |r| Show InChI InChI=1S/C27H24N4O6/c28-15-16-4-5-17(2-1-3-24(32)33)21(12-16)30-26(35)20-14-27(20)9-11-36-22-7-6-18(13-19(22)27)25(34)31-23-8-10-29-37-23/h4-8,10,12-13,20H,1-3,9,11,14H2,(H,30,35)(H,31,34)(H,32,33)/t20-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
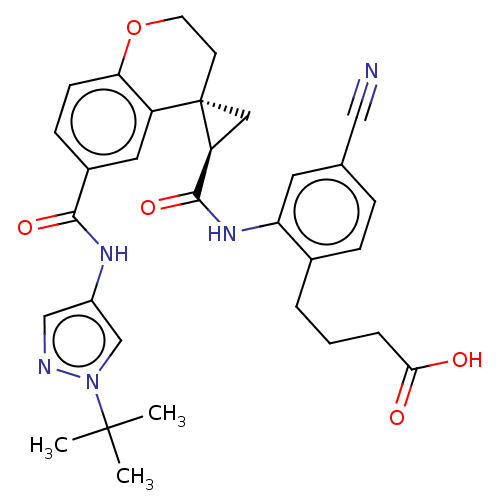
(Homo sapiens (Human)) | BDBM285476
(US10077247, Example 2-39)Show SMILES CC(C)(C)n1cc(NC(=O)c2ccc3OCC[C@]4(C[C@H]4C(=O)Nc4cc(ccc4CCCC(O)=O)C#N)c3c2)cn1 |r| Show InChI InChI=1S/C31H33N5O5/c1-30(2,3)36-18-22(17-33-36)34-28(39)21-9-10-26-23(14-21)31(11-12-41-26)15-24(31)29(40)35-25-13-19(16-32)7-8-20(25)5-4-6-27(37)38/h7-10,13-14,17-18,24H,4-6,11-12,15H2,1-3H3,(H,34,39)(H,35,40)(H,37,38)/t24-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285474

(4-[4-Cyano-2-({[(2′R,4S)-6-(1,2-oxazol-3-ylc...)Show SMILES OC(=O)CCCc1ccc(cc1NC(=O)[C@@H]1C[C@]11CCOc2ccc(cc12)C(=O)Nc1ccon1)C#N |r| Show InChI InChI=1S/C27H24N4O6/c28-15-16-4-5-17(2-1-3-24(32)33)21(12-16)29-26(35)20-14-27(20)9-11-36-22-7-6-18(13-19(22)27)25(34)30-23-8-10-37-31-23/h4-8,10,12-13,20H,1-3,9,11,14H2,(H,29,35)(H,32,33)(H,30,31,34)/t20-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285489

(4-[4-Cyano-2-({[(2′R,4S)-6-(6-methoxy-3-pyri...)Show SMILES COc1ccc(cn1)-c1ccc2OCC[C@]3(C[C@H]3C(=O)Nc3cc(ccc3CCCC(O)=O)C#N)c2c1 |r| Show InChI InChI=1S/C29H27N3O5/c1-36-26-10-8-21(17-31-26)20-7-9-25-22(14-20)29(11-12-37-25)15-23(29)28(35)32-24-13-18(16-30)5-6-19(24)3-2-4-27(33)34/h5-10,13-14,17,23H,2-4,11-12,15H2,1H3,(H,32,35)(H,33,34)/t23-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285495
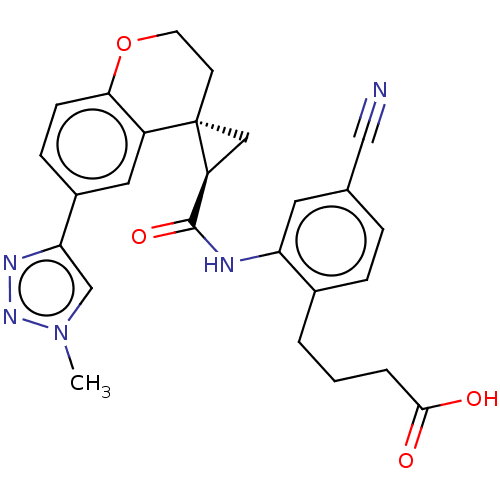
(4-[4-Cyano-2-({[(2′R,4S)-6-(1-methyl-1H-1,2,...)Show SMILES Cn1cc(nn1)-c1ccc2OCC[C@]3(C[C@H]3C(=O)Nc3cc(ccc3CCCC(O)=O)C#N)c2c1 |r| Show InChI InChI=1S/C26H25N5O4/c1-31-15-22(29-30-31)18-7-8-23-19(12-18)26(9-10-35-23)13-20(26)25(34)28-21-11-16(14-27)5-6-17(21)3-2-4-24(32)33/h5-8,11-12,15,20H,2-4,9-10,13H2,1H3,(H,28,34)(H,32,33)/t20-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285462

(US10077247, Example 2-3)Show SMILES CC(C)(C)NC(=O)c1ccc2OCC[C@]3(C[C@H]3C(=O)Nc3cc(ccc3CCCC(O)=O)C#N)c2c1 |r| Show InChI InChI=1S/C28H31N3O5/c1-27(2,3)31-25(34)19-9-10-23-20(14-19)28(11-12-36-23)15-21(28)26(35)30-22-13-17(16-29)7-8-18(22)5-4-6-24(32)33/h7-10,13-14,21H,4-6,11-12,15H2,1-3H3,(H,30,35)(H,31,34)(H,32,33)/t21-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285479
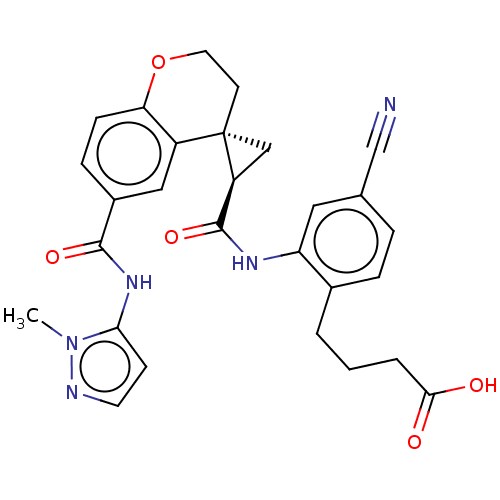
(4-{4-Cyano-2-[({(2′R,4S)-6-[(1-methyl-1H-pyr...)Show SMILES Cn1nccc1NC(=O)c1ccc2OCC[C@]3(C[C@H]3C(=O)Nc3cc(ccc3CCCC(O)=O)C#N)c2c1 |r| Show InChI InChI=1S/C28H27N5O5/c1-33-24(9-11-30-33)32-26(36)19-7-8-23-20(14-19)28(10-12-38-23)15-21(28)27(37)31-22-13-17(16-29)5-6-18(22)3-2-4-25(34)35/h5-9,11,13-14,21H,2-4,10,12,15H2,1H3,(H,31,37)(H,32,36)(H,34,35)/t21-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285471

(4-{4-Cyano-2-[({(2′R,4S)-6-[(2,2-difluoroeth...)Show SMILES OC(=O)CCCc1ccc(cc1NC(=O)[C@@H]1C[C@]11CCOc2ccc(cc12)C(=O)NCC(F)F)C#N |r| Show InChI InChI=1S/C26H25F2N3O5/c27-22(28)14-30-24(34)17-6-7-21-18(11-17)26(8-9-36-21)12-19(26)25(35)31-20-10-15(13-29)4-5-16(20)2-1-3-23(32)33/h4-7,10-11,19,22H,1-3,8-9,12,14H2,(H,30,34)(H,31,35)(H,32,33)/t19-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285486

(4-[4-Cyano-2-({[(2′R,4S)-6-(3-pyridinyl)-2,3...)Show SMILES OC(=O)CCCc1ccc(cc1NC(=O)[C@@H]1C[C@]11CCOc2ccc(cc12)-c1cccnc1)C#N |r| Show InChI InChI=1S/C28H25N3O4/c29-16-18-6-7-19(3-1-5-26(32)33)24(13-18)31-27(34)23-15-28(23)10-12-35-25-9-8-20(14-22(25)28)21-4-2-11-30-17-21/h2,4,6-9,11,13-14,17,23H,1,3,5,10,12,15H2,(H,31,34)(H,32,33)/t23-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082340
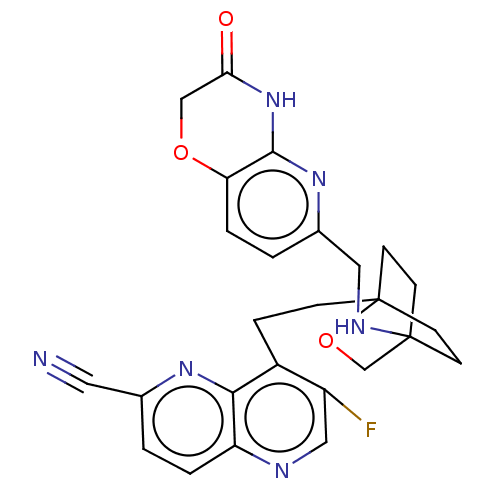
(CHEMBL3422947)Show SMILES Fc1cnc2ccc(nc2c1CCC12CCC(CC1)(CO2)NCc1ccc2OCC(=O)Nc2n1)C#N |(-12.24,-4.65,;-12.56,-5.84,;-14.04,-6.24,;-14.44,-7.72,;-13.36,-8.81,;-13.75,-10.3,;-12.66,-11.41,;-11.17,-11.01,;-10.78,-9.5,;-11.87,-8.41,;-11.47,-6.93,;-9.98,-6.52,;-9.58,-5.03,;-8.11,-4.64,;-7.75,-3.13,;-6.28,-2.7,;-5.35,-3.86,;-5.52,-5.26,;-6.99,-5.7,;-6.24,-4.82,;-7.05,-3.51,;-4.02,-3.09,;-4.02,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-10.08,-12.1,;-9.21,-12.97,)| Show InChI InChI=1S/C26H25FN6O3/c27-19-13-29-20-3-1-16(11-28)31-23(20)18(19)5-6-26-9-7-25(8-10-26,15-36-26)30-12-17-2-4-21-24(32-17)33-22(34)14-35-21/h1-4,13,30H,5-10,12,14-15H2,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285466
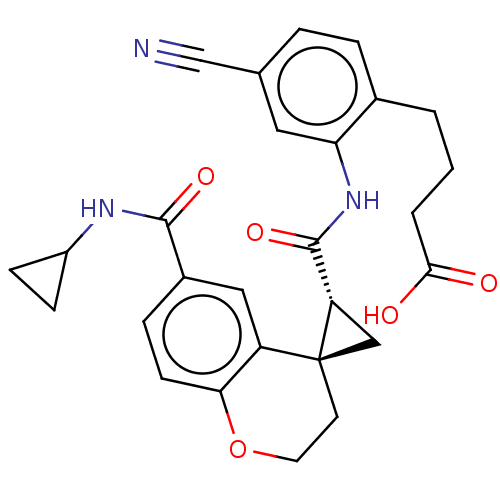
(4-[4-cyano-2-({[(2′R,4S)-6-(cyclopropylcarba...)Show SMILES OC(=O)CCCc1ccc(cc1NC(=O)[C@@H]1C[C@]11CCOc2ccc(cc12)C(=O)NC1CC1)C#N |r| Show InChI InChI=1S/C27H27N3O5/c28-15-16-4-5-17(2-1-3-24(31)32)22(12-16)30-26(34)21-14-27(21)10-11-35-23-9-6-18(13-20(23)27)25(33)29-19-7-8-19/h4-6,9,12-13,19,21H,1-3,7-8,10-11,14H2,(H,29,33)(H,30,34)(H,31,32)/t21-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285496
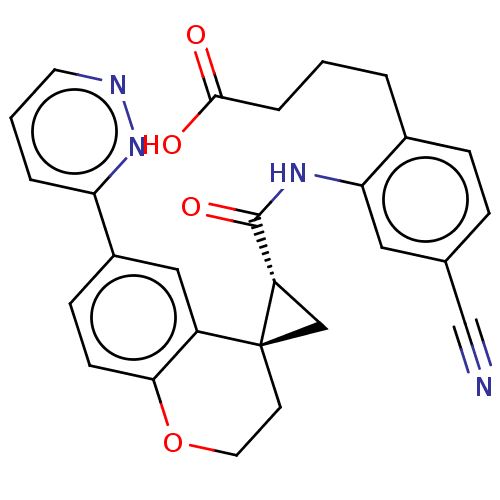
(4-[4-Cyano-2-({[(2′R,4S)-6-(3-pyridazinyl)-2...)Show SMILES OC(=O)CCCc1ccc(cc1NC(=O)[C@@H]1C[C@]11CCOc2ccc(cc12)-c1cccnn1)C#N |r| Show InChI InChI=1S/C27H24N4O4/c28-16-17-6-7-18(3-1-5-25(32)33)23(13-17)30-26(34)21-15-27(21)10-12-35-24-9-8-19(14-20(24)27)22-4-2-11-29-31-22/h2,4,6-9,11,13-14,21H,1,3,5,10,12,15H2,(H,30,34)(H,32,33)/t21-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082428
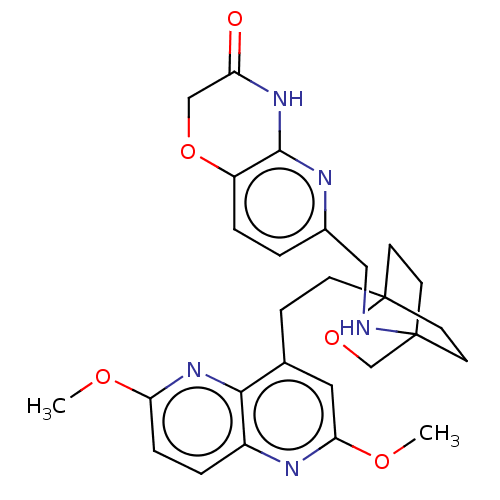
(CHEMBL3422969)Show SMILES COc1ccc2nc(OC)cc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;4.01,-1.54,;5.07,-.92,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H31N5O5/c1-34-22-6-4-19-24(32-22)17(13-23(30-19)35-2)7-8-27-11-9-26(10-12-27,16-37-27)28-14-18-3-5-20-25(29-18)31-21(33)15-36-20/h3-6,13,28H,7-12,14-16H2,1-2H3,(H,29,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082389

(CHEMBL3422963)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1Cl |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C26H28ClN5O4/c1-34-24-18(27)12-19-22(32-24)16(5-11-28-19)4-6-26-9-7-25(8-10-26,15-36-26)29-13-17-2-3-20-23(30-17)31-21(33)14-35-20/h2-3,5,11-12,29H,4,6-10,13-15H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285512
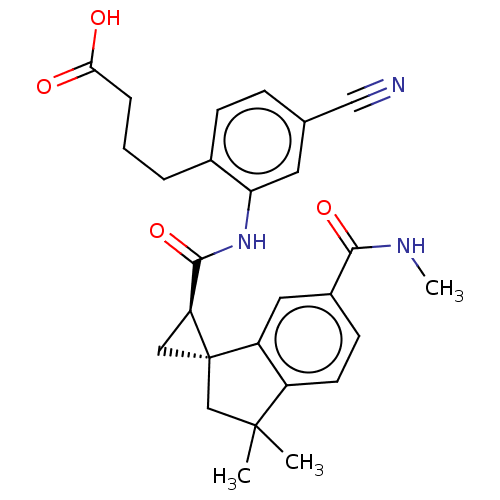
(4-[4-Cyano-2-({[(1S,2R)-3′,3′-dimethyl...)Show SMILES CNC(=O)c1ccc2c(c1)[C@@]1(C[C@H]1C(=O)Nc1cc(ccc1CCCC(O)=O)C#N)CC2(C)C |r| Show InChI InChI=1S/C27H29N3O4/c1-26(2)15-27(20-12-18(24(33)29-3)9-10-19(20)26)13-21(27)25(34)30-22-11-16(14-28)7-8-17(22)5-4-6-23(31)32/h7-12,21H,4-6,13,15H2,1-3H3,(H,29,33)(H,30,34)(H,31,32)/t21-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM285469
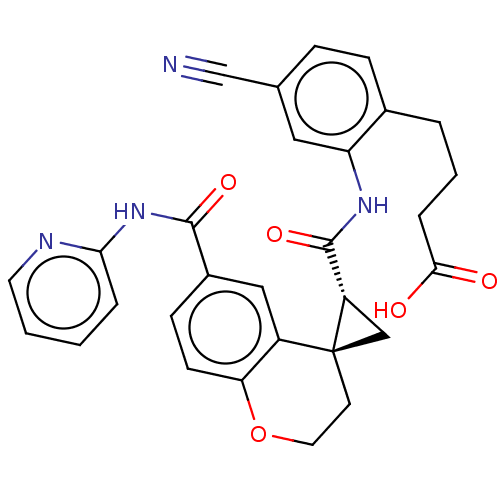
(4-[4-Cyano-2-({[(2′R,4S)-6-(2-pyridinylcarba...)Show SMILES OC(=O)CCCc1ccc(cc1NC(=O)[C@@H]1C[C@]11CCOc2ccc(cc12)C(=O)Nc1ccccn1)C#N |r| Show InChI InChI=1S/C29H26N4O5/c30-17-18-7-8-19(4-3-6-26(34)35)23(14-18)32-28(37)22-16-29(22)11-13-38-24-10-9-20(15-21(24)29)27(36)33-25-5-1-2-12-31-25/h1-2,5,7-10,12,14-15,22H,3-4,6,11,13,16H2,(H,32,37)(H,34,35)(H,31,33,36)/t22-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | 25 |
ONO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
EP4 Antagonistic Activity Measurement Experiment Using Prostanoid Receptor Subtype Expressing CellsCHO cells expressing rat EP4 receptor subtypes wer... |
US Patent US10077247 (2018)
BindingDB Entry DOI: 10.7270/Q22J6DWN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data