

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50316153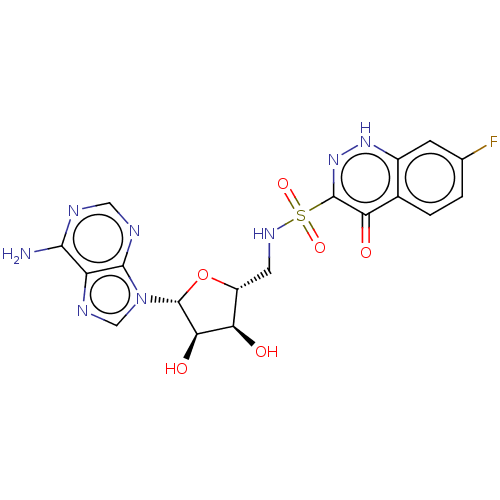 (CHEMBL4299745) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant salicylate ligase MbtA expressed in Escherichia coli using salicylic acid as substrate after 20 ... | ACS Med Chem Lett 9: 386-391 (2018) Article DOI: 10.1021/acsmedchemlett.8b00090 BindingDB Entry DOI: 10.7270/Q2RR21SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50316151 (CHEMBL4299747) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant salicylate ligase MbtA expressed in Escherichia coli using salicylic acid as substrate after 20 ... | ACS Med Chem Lett 9: 386-391 (2018) Article DOI: 10.1021/acsmedchemlett.8b00090 BindingDB Entry DOI: 10.7270/Q2RR21SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50316152 (CHEMBL4299749) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant salicylate ligase MbtA expressed in Escherichia coli using salicylic acid as substrate after 20 ... | ACS Med Chem Lett 9: 386-391 (2018) Article DOI: 10.1021/acsmedchemlett.8b00090 BindingDB Entry DOI: 10.7270/Q2RR21SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50316154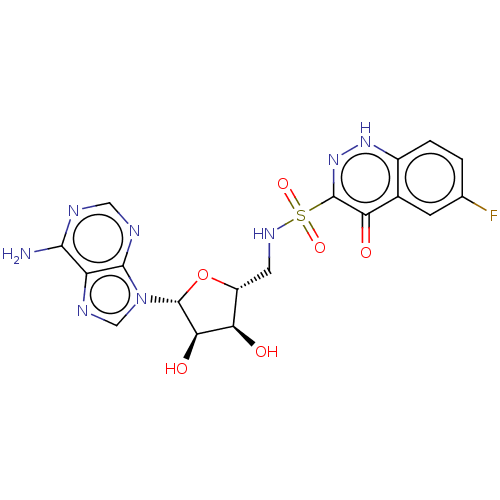 (CHEMBL4299748) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant salicylate ligase MbtA expressed in Escherichia coli using salicylic acid as substrate after 20 ... | ACS Med Chem Lett 9: 386-391 (2018) Article DOI: 10.1021/acsmedchemlett.8b00090 BindingDB Entry DOI: 10.7270/Q2RR21SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD32 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565056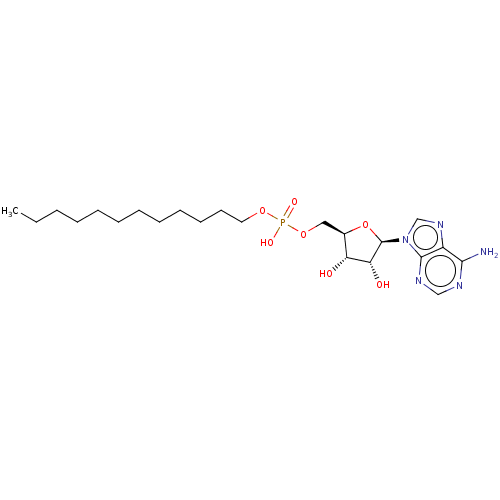 (CHEMBL4783416) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL32 expressed in Escherichia coli BL21(DE3) cells incubated for 2 hrs in presence of [C14] fatty ac... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50316149 (CHEMBL4168282) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant salicylate ligase MbtA expressed in Escherichia coli using salicylic acid as substrate after 20 ... | ACS Med Chem Lett 9: 386-391 (2018) Article DOI: 10.1021/acsmedchemlett.8b00090 BindingDB Entry DOI: 10.7270/Q2RR21SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565085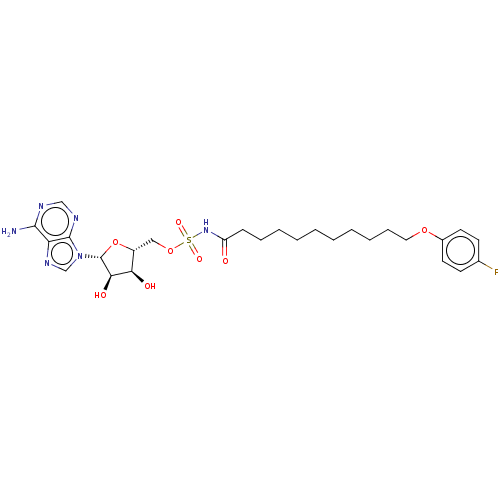 (CHEMBL4795814) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565089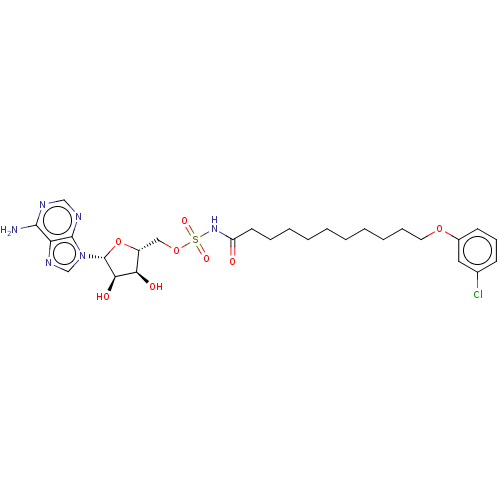 (CHEMBL4778102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565063 (CHEMBL4789026) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565075 (CHEMBL4784453) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565062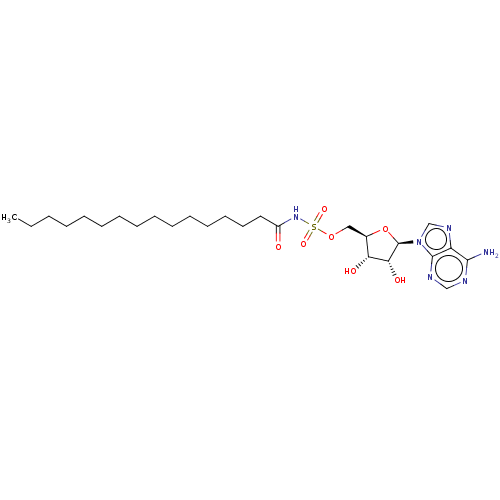 (CHEMBL4794550) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50456068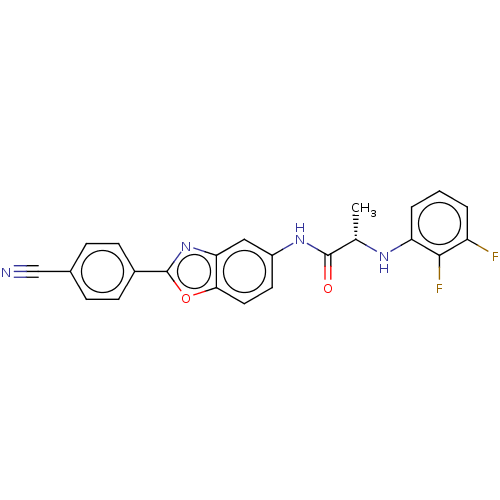 (CHEMBL4208344) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis IMPDH2 Y487C mutant using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565094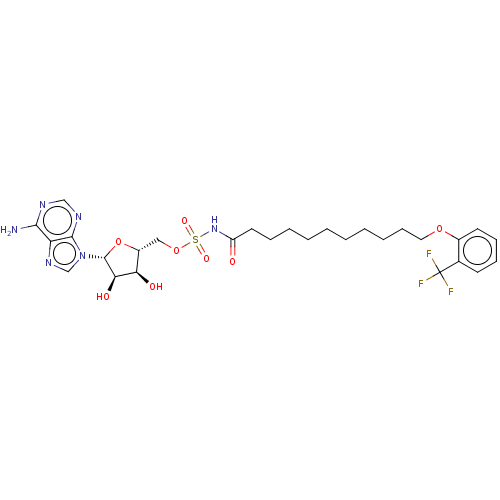 (CHEMBL4793140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565061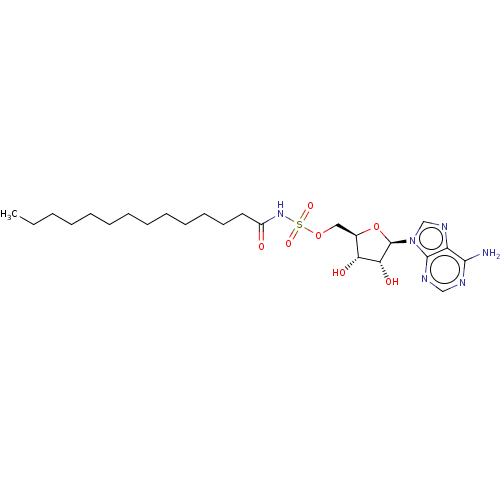 (CHEMBL4793406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565055 (CHEMBL1213367) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv His-tagged FAAL28 expressed in Escherichia coli | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565087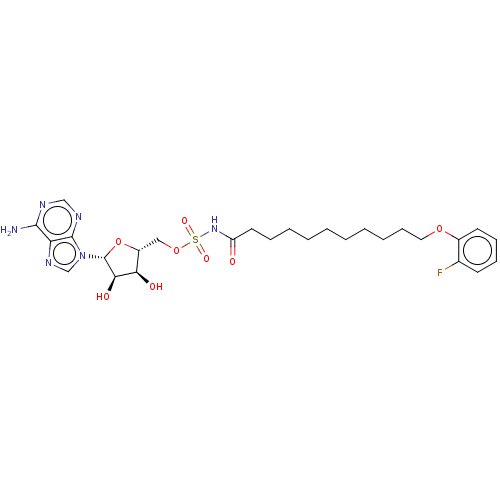 (CHEMBL4796346) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565086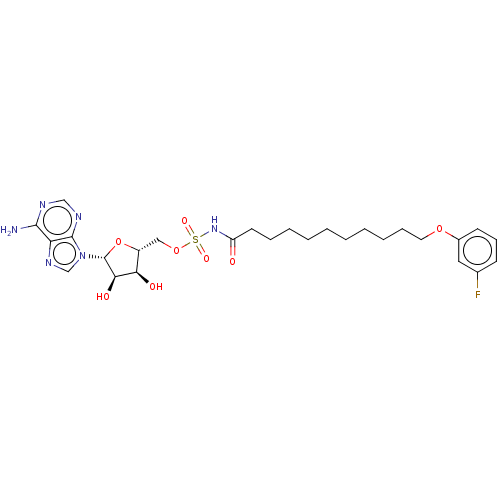 (CHEMBL4793079) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565096 (CHEMBL4783091) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565099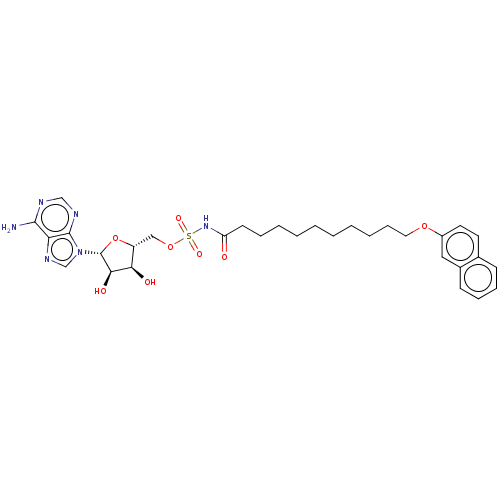 (CHEMBL4788041) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456074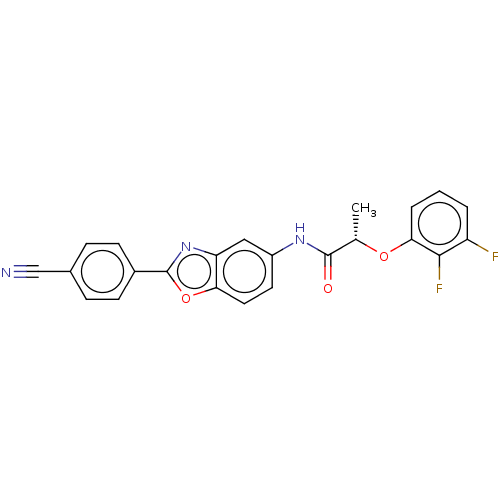 (CHEMBL4202438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565088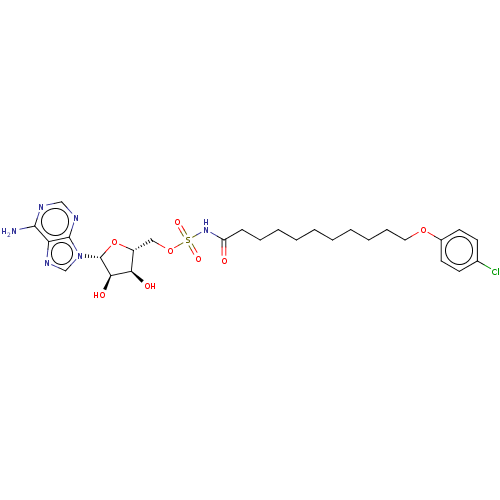 (CHEMBL4788163) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456086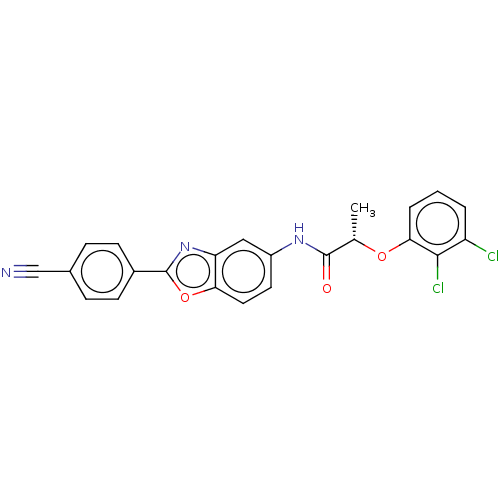 (CHEMBL4204706) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Medium/long-chain-fatty-acid--CoA/3-oxocholest-4-en-26-oate--CoA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565063 (CHEMBL4789026) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FACL19 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565082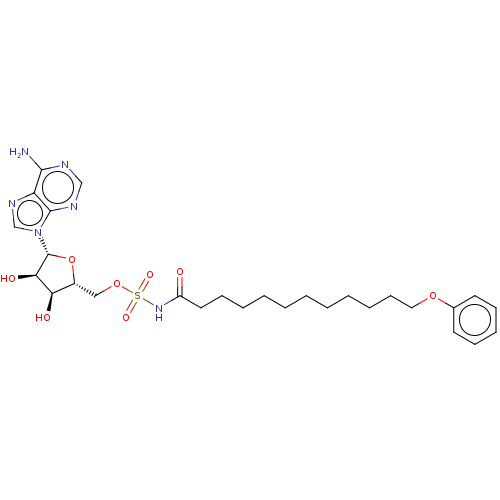 (CHEMBL4786161) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456092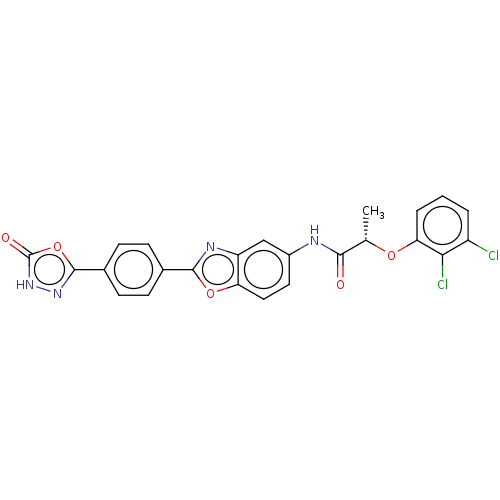 (CHEMBL4218122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565100 (CHEMBL4779275) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456097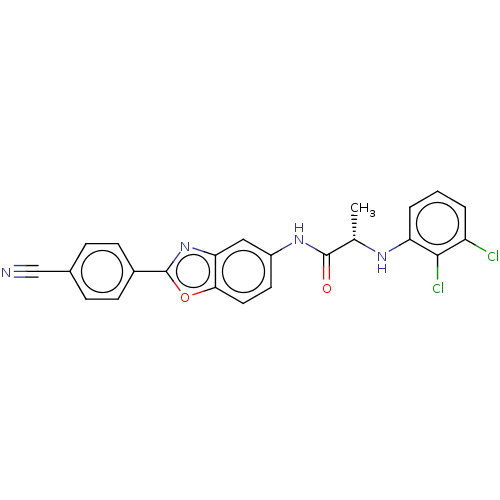 (CHEMBL4217858) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565090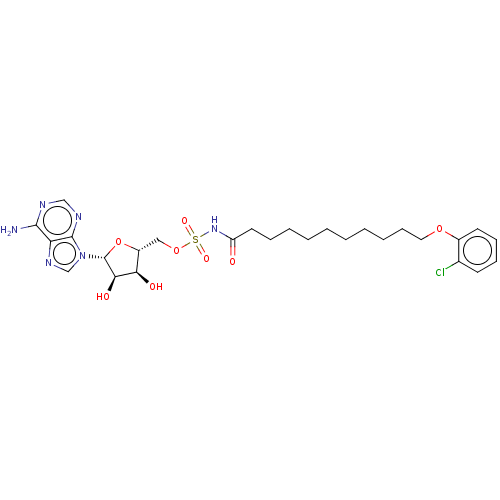 (CHEMBL4794608) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565091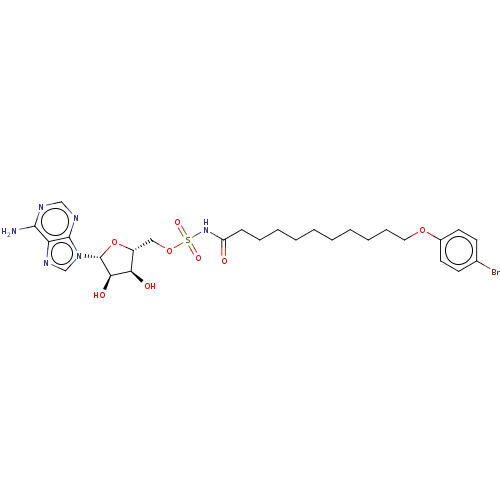 (CHEMBL4786635) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain-fatty-acid--AMP ligase FadD28 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565093 (CHEMBL4785012) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv FAAL28 Spectra expressed in Escherichia coli BL21(DE3) cells assessed as cleavage of MEsG by spectrosc... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Medium/long-chain-fatty-acid--CoA/3-oxocholest-4-en-26-oate--CoA ligase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50565055 (CHEMBL1213367) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis H37Rv His-tagged FACL19 expressed in Escherichia coli | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112408 BindingDB Entry DOI: 10.7270/Q2Z60SS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456062 (CHEMBL4204618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456063 (CHEMBL4215874) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50456064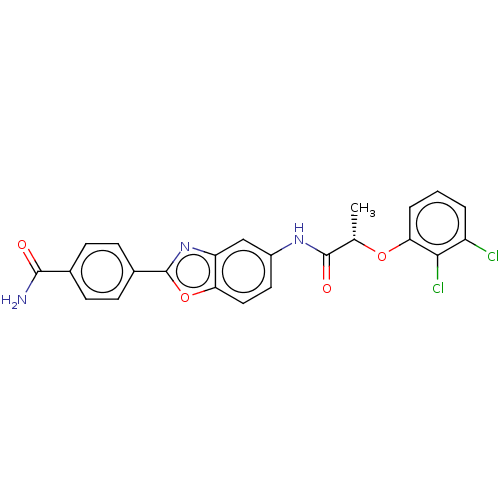 (CHEMBL4210256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human IMPDH2 using IMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456065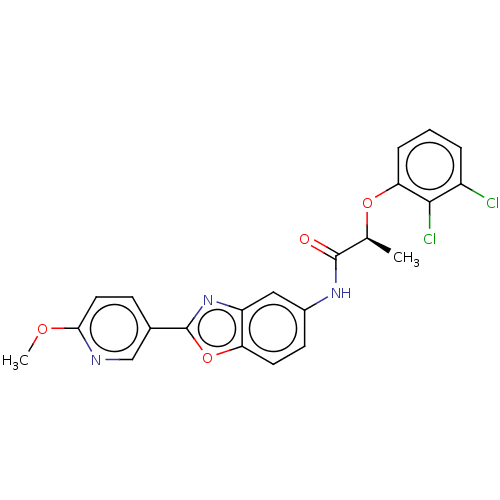 (CHEMBL4204401) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456066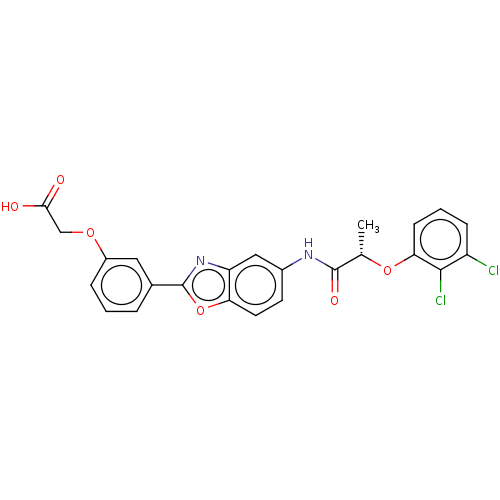 (CHEMBL4209336) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456067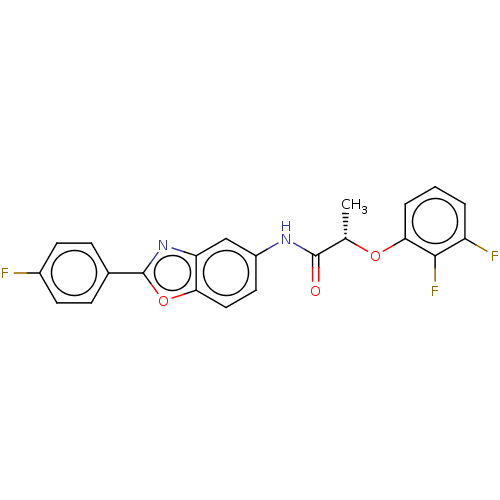 (CHEMBL4203696) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456069 (CHEMBL4206398) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456070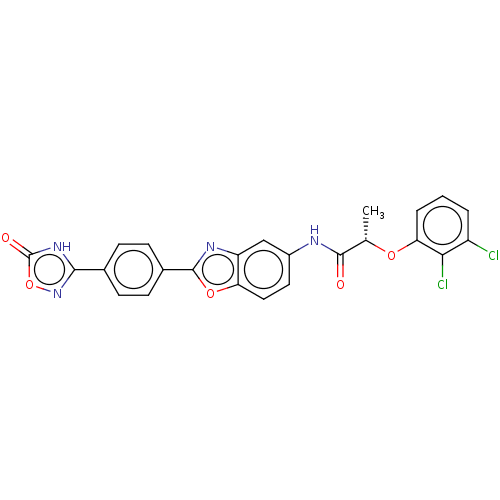 (CHEMBL4205894) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456071 (CHEMBL4210769) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456072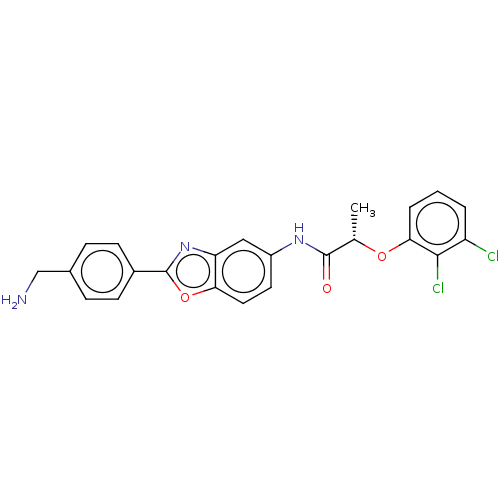 (CHEMBL4209120) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456073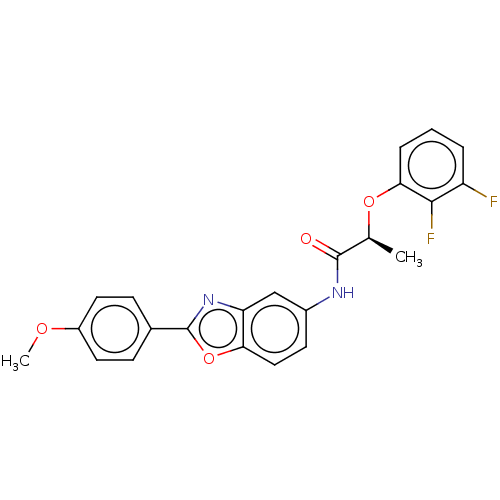 (CHEMBL4204321) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456074 (CHEMBL4202438) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456075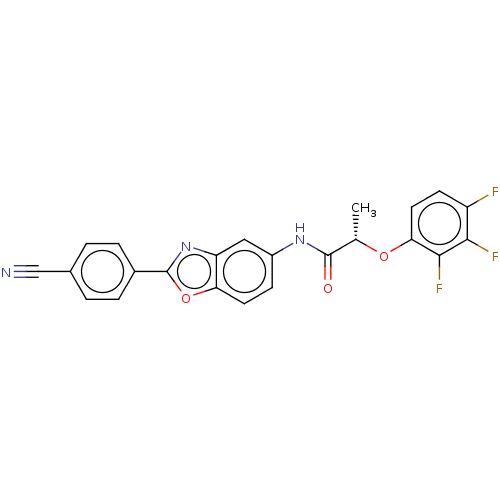 (CHEMBL4216354) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456076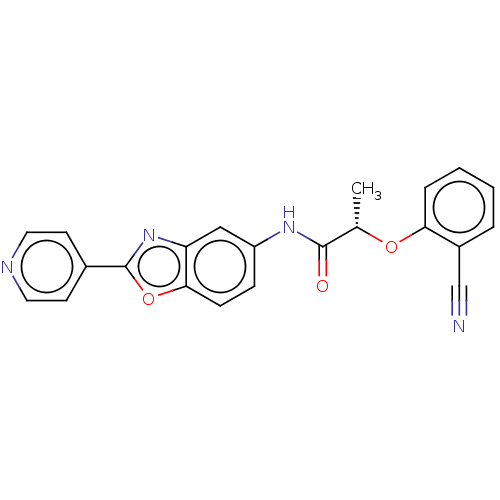 (CHEMBL4215758) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456077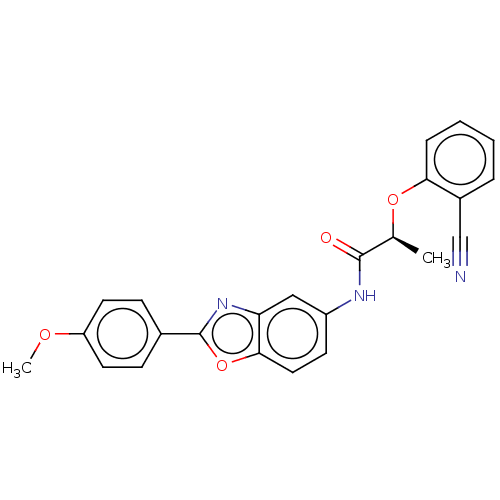 (CHEMBL4211469) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456078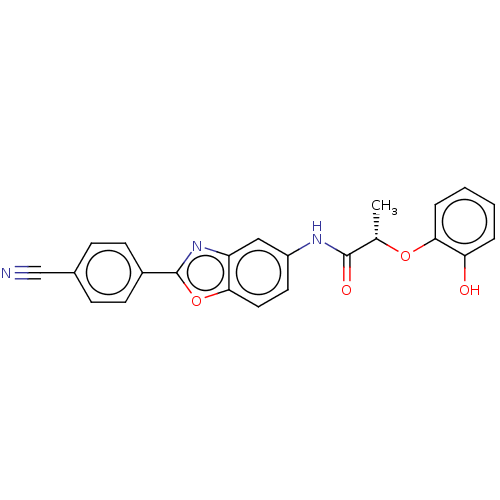 (CHEMBL4207934) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456079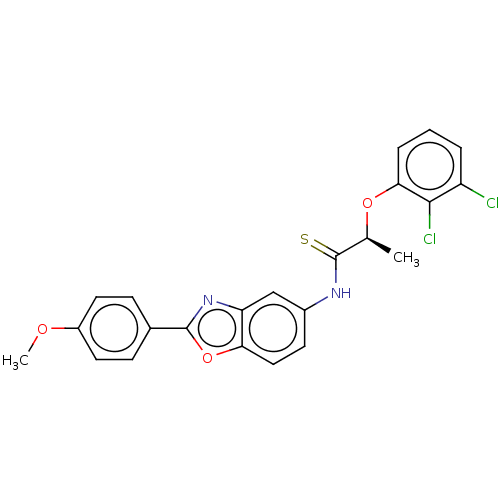 (CHEMBL4209344) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456080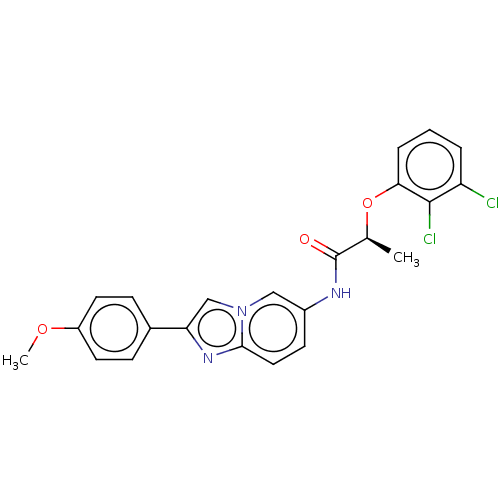 (CHEMBL4215780) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GMP reductase 2 (Homo sapiens) | BDBM50456081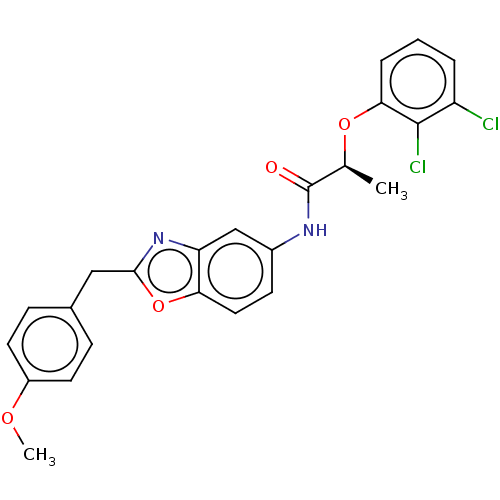 (CHEMBL4210836) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Allergy and Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of human GMPR2 using GMP as substrate by spectrophotometric method | J Med Chem 61: 4739-4756 (2018) Article DOI: 10.1021/acs.jmedchem.7b01839 BindingDB Entry DOI: 10.7270/Q29889M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 211 total ) | Next | Last >> |