 Found 188 hits with Last Name = 'wadsworth' and Initial = 'hj'
Found 188 hits with Last Name = 'wadsworth' and Initial = 'hj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Translocator protein
(Rattus norvegicus (rat)) | BDBM253021

(US9481685, 4)Show SMILES CCN(CC)C(=O)C1Sc2c(F)cccc2-c2c1c1ccccc1n2CCF Show InChI InChI=1S/C22H22F2N2OS/c1-3-25(4-2)22(27)21-18-14-8-5-6-11-17(14)26(13-12-23)19(18)15-9-7-10-16(24)20(15)28-21/h5-11,21H,3-4,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GE Healthcare Limited
US Patent
| Assay Description
The compounds were screened for their affinity for PBR using a method adapted from Le Fur et al (Life Sci. 1983; USA 33: 449-57).The compounds to be ... |
US Patent US9481685 (2016)
BindingDB Entry DOI: 10.7270/Q25M64NT |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM253022
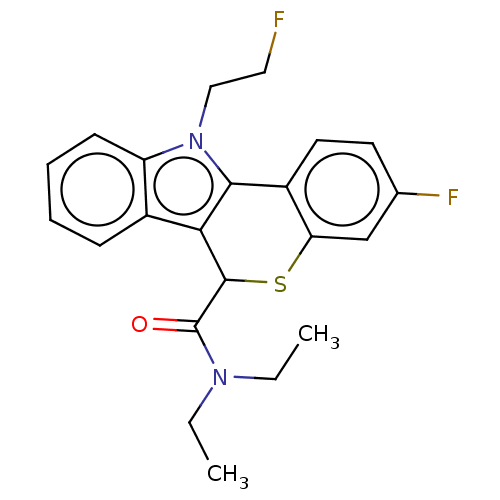
(US9481685, 5)Show SMILES CCN(CC)C(=O)C1Sc2cc(F)ccc2-c2c1c1ccccc1n2CCF Show InChI InChI=1S/C22H22F2N2OS/c1-3-25(4-2)22(27)21-19-15-7-5-6-8-17(15)26(12-11-23)20(19)16-10-9-14(24)13-18(16)28-21/h5-10,13,21H,3-4,11-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GE Healthcare Limited
US Patent
| Assay Description
The compounds were screened for their affinity for PBR using a method adapted from Le Fur et al (Life Sci. 1983; USA 33: 449-57).The compounds to be ... |
US Patent US9481685 (2016)
BindingDB Entry DOI: 10.7270/Q25M64NT |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM253019

(US9481685, 1)Show SMILES CCN(CC)C(=O)C1Sc2ccccc2-c2c1c1cc(OC)ccc1n2CCF Show InChI InChI=1S/C23H25FN2O2S/c1-4-25(5-2)23(27)22-20-17-14-15(28-3)10-11-18(17)26(13-12-24)21(20)16-8-6-7-9-19(16)29-22/h6-11,14,22H,4-5,12-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GE Healthcare Limited
US Patent
| Assay Description
The compounds were screened for their affinity for PBR using a method adapted from Le Fur et al (Life Sci. 1983; USA 33: 449-57).The compounds to be ... |
US Patent US9481685 (2016)
BindingDB Entry DOI: 10.7270/Q25M64NT |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM253024
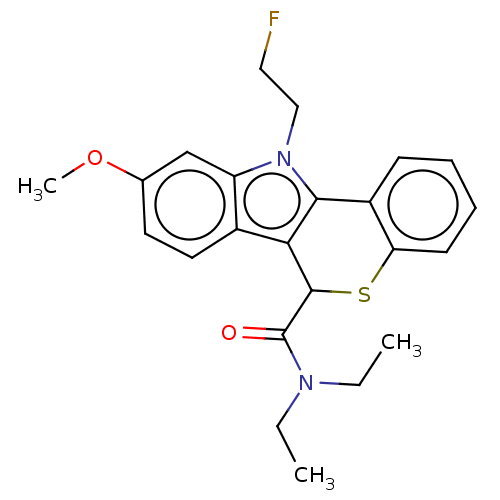
(US9481685, 2)Show SMILES CCN(CC)C(=O)C1Sc2ccccc2-c2c1c1ccc(OC)cc1n2CCF Show InChI InChI=1S/C23H25FN2O2S/c1-4-25(5-2)23(27)22-20-16-11-10-15(28-3)14-18(16)26(13-12-24)21(20)17-8-6-7-9-19(17)29-22/h6-11,14,22H,4-5,12-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GE Healthcare Limited
US Patent
| Assay Description
The compounds were screened for their affinity for PBR using a method adapted from Le Fur et al (Life Sci. 1983; USA 33: 449-57).The compounds to be ... |
US Patent US9481685 (2016)
BindingDB Entry DOI: 10.7270/Q25M64NT |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM253023

(US9481685, 6)Show SMILES CCOc1ccc2n(CCF)c-3c(C(Sc4ccccc-34)C(=O)N(CC)CC)c2c1 Show InChI InChI=1S/C24H27FN2O2S/c1-4-26(5-2)24(28)23-21-18-15-16(29-6-3)11-12-19(18)27(14-13-25)22(21)17-9-7-8-10-20(17)30-23/h7-12,15,23H,4-6,13-14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GE Healthcare Limited
US Patent
| Assay Description
The compounds were screened for their affinity for PBR using a method adapted from Le Fur et al (Life Sci. 1983; USA 33: 449-57).The compounds to be ... |
US Patent US9481685 (2016)
BindingDB Entry DOI: 10.7270/Q25M64NT |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50363527
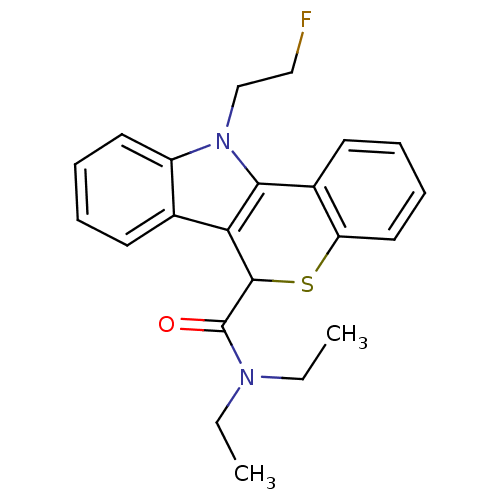
(CHEMBL1945412 | US9481685, [18F]FE-PBR)Show InChI InChI=1S/C22H23FN2OS/c1-3-24(4-2)22(26)21-19-15-9-5-7-11-17(15)25(14-13-23)20(19)16-10-6-8-12-18(16)27-21/h5-12,21H,3-4,13-14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| US Patent
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GE Healthcare Limited
US Patent
| Assay Description
The compounds were screened for their affinity for PBR using a method adapted from Le Fur et al (Life Sci. 1983; USA 33: 449-57).The compounds to be ... |
US Patent US9481685 (2016)
BindingDB Entry DOI: 10.7270/Q25M64NT |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM253020

(US9481685, 3)Show SMILES CCN(CC)C(=O)C1Sc2ccccc2-c2c1c1cccc(OC)c1n2CCF Show InChI InChI=1S/C23H25FN2O2S/c1-4-25(5-2)23(27)22-19-16-10-8-11-17(28-3)20(16)26(14-13-24)21(19)15-9-6-7-12-18(15)29-22/h6-12,22H,4-5,13-14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GE Healthcare Limited
US Patent
| Assay Description
The compounds were screened for their affinity for PBR using a method adapted from Le Fur et al (Life Sci. 1983; USA 33: 449-57).The compounds to be ... |
US Patent US9481685 (2016)
BindingDB Entry DOI: 10.7270/Q25M64NT |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM253018

(US9481685, 7)Show SMILES CCN(CC)C(=O)C1Sc2ccccc2-c2c1c1c(OC)cccc1n2CCF Show InChI InChI=1S/C23H25FN2O2S/c1-4-25(5-2)23(27)22-20-19-16(10-8-11-17(19)28-3)26(14-13-24)21(20)15-9-6-7-12-18(15)29-22/h6-12,22H,4-5,13-14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
GE Healthcare Limited
US Patent
| Assay Description
The compounds were screened for their affinity for PBR using a method adapted from Le Fur et al (Life Sci. 1983; USA 33: 449-57).The compounds to be ... |
US Patent US9481685 (2016)
BindingDB Entry DOI: 10.7270/Q25M64NT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229676

(CHEMBL78697)Show InChI InChI=1S/C7H12N6/c8-7-9-11-13(10-7)6-4-12-2-1-5(6)3-12/h5-6H,1-4H2,(H2,8,10)/t5-,6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229669
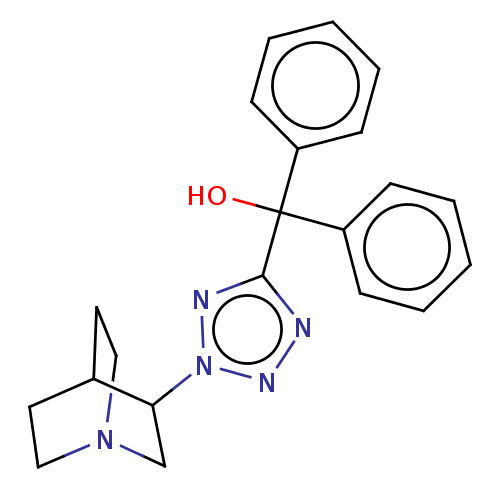
(CHEMBL309432)Show SMILES OC(c1nnn(n1)C1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(9.08,-4.44,;7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,;8.45,-3.21,;7.81,-1.81,;8.71,-.56,;10.25,-.72,;10.88,-2.13,;9.98,-3.37,;8.16,-5.85,;7.26,-7.1,;7.87,-8.5,;9.41,-8.66,;10.31,-7.41,;9.69,-6.01,)| Show InChI InChI=1S/C21H23N5O/c27-21(17-7-3-1-4-8-17,18-9-5-2-6-10-18)20-22-24-26(23-20)19-15-25-13-11-16(19)12-14-25/h1-10,16,19,27H,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]quinuclidinyl benzilate (QNB) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229528

(CHEMBL93406)Show InChI InChI=1S/C9H13N3O/c1-6-10-9(13-11-6)8-5-12-3-2-7(8)4-12/h7-8H,2-5H2,1H3/t7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for central muscarinic acetylcholine receptor affinity to displace [3H]oxotremorine-M from rat cerebral cortex; Valu... |
J Med Chem 34: 2726-35 (1991)
BindingDB Entry DOI: 10.7270/Q2BG2R7J |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229684
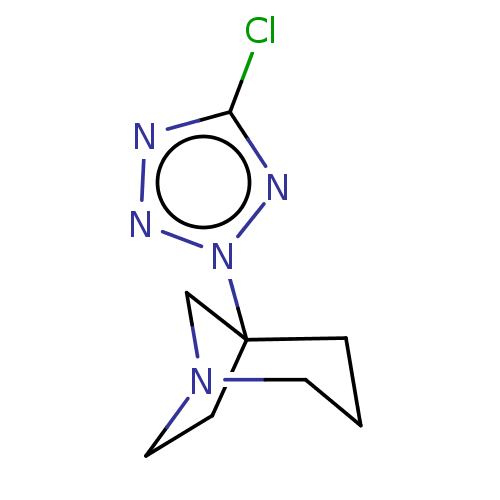
(CHEMBL78615)Show InChI InChI=1S/C8H12ClN5/c9-7-10-12-14(11-7)8-2-1-4-13(6-8)5-3-8/h1-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229669

(CHEMBL309432)Show SMILES OC(c1nnn(n1)C1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(9.08,-4.44,;7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,;8.45,-3.21,;7.81,-1.81,;8.71,-.56,;10.25,-.72,;10.88,-2.13,;9.98,-3.37,;8.16,-5.85,;7.26,-7.1,;7.87,-8.5,;9.41,-8.66,;10.31,-7.41,;9.69,-6.01,)| Show InChI InChI=1S/C21H23N5O/c27-21(17-7-3-1-4-8-17,18-9-5-2-6-10-18)20-22-24-26(23-20)19-15-25-13-11-16(19)12-14-25/h1-10,16,19,27H,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229617
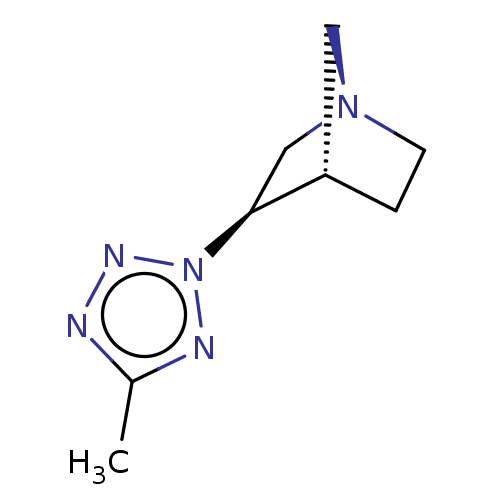
(CHEMBL311799)Show InChI InChI=1S/C8H13N5/c1-6-9-11-13(10-6)8-5-12-3-2-7(8)4-12/h7-8H,2-5H2,1H3/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229681
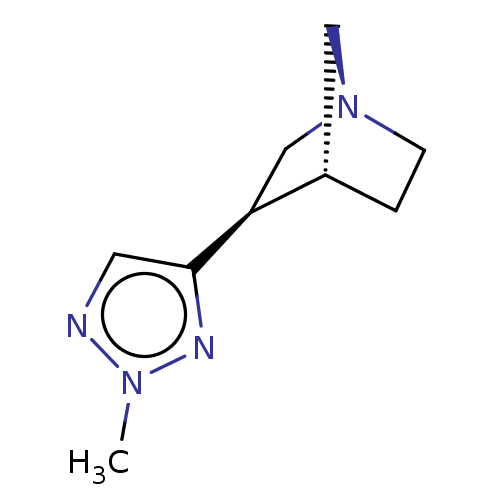
(CHEMBL79040)Show InChI InChI=1S/C9H14N4/c1-12-10-4-9(11-12)8-6-13-3-2-7(8)5-13/h4,7-8H,2-3,5-6H2,1H3/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229685
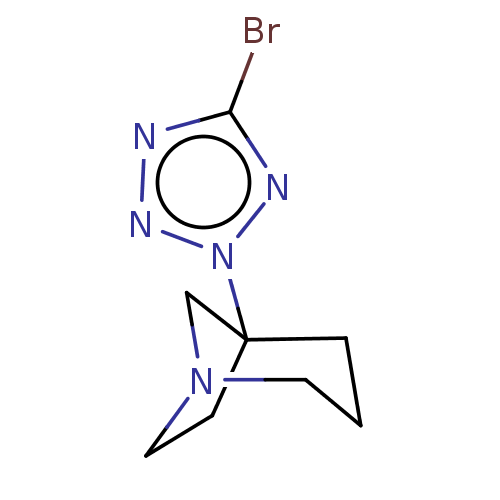
(CHEMBL311212)Show InChI InChI=1S/C8H12BrN5/c9-7-10-12-14(11-7)8-2-1-4-13(6-8)5-3-8/h1-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex Muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229678
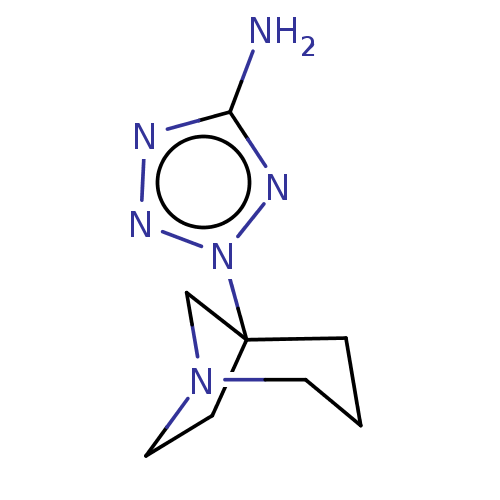
(CHEMBL80723)Show InChI InChI=1S/C8H14N6/c9-7-10-12-14(11-7)8-2-1-4-13(6-8)5-3-8/h1-6H2,(H2,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229696

(CHEMBL419659)Show InChI InChI=1S/C7H10BrN5/c8-7-9-11-13(10-7)6-4-12-2-1-5(6)3-12/h5-6H,1-4H2/t5-,6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229694
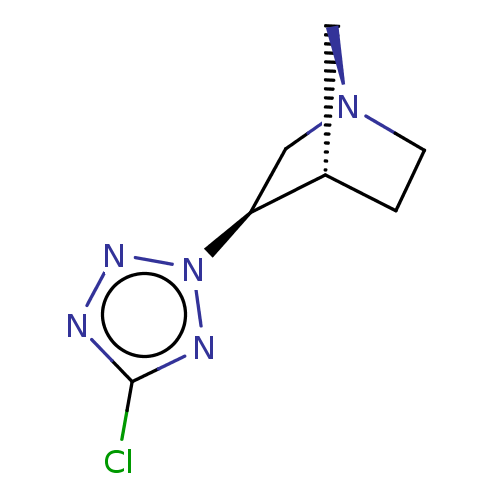
(CHEMBL81481)Show InChI InChI=1S/C7H10ClN5/c8-7-9-11-13(10-7)6-4-12-2-1-5(6)3-12/h5-6H,1-4H2/t5-,6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229623

(CHEMBL81281)Show InChI InChI=1S/C8H13N5/c1-12-10-8(9-11-12)7-5-13-3-2-6(7)4-13/h6-7H,2-5H2,1H3/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for central muscarinic acetylcholine receptor affinity to displace [3H]oxotremorine-M from rat cerebral cortex; Valu... |
J Med Chem 34: 2726-35 (1991)
BindingDB Entry DOI: 10.7270/Q2BG2R7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229675
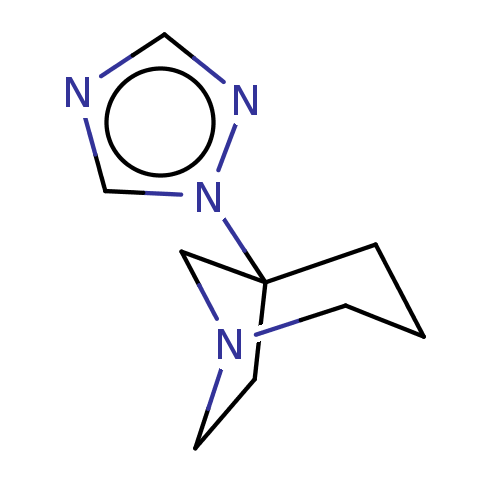
(CHEMBL309638)Show InChI InChI=1S/C9H14N4/c1-2-9(13-8-10-7-11-13)3-5-12(4-1)6-9/h7-8H,1-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM10759

(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for its binding affinity to muscarinic acetylcholine receptor from rat cortical homogenates using [3H]OXO-M as radioliga... |
J Med Chem 35: 1280-90 (1992)
BindingDB Entry DOI: 10.7270/Q2G1632Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229692
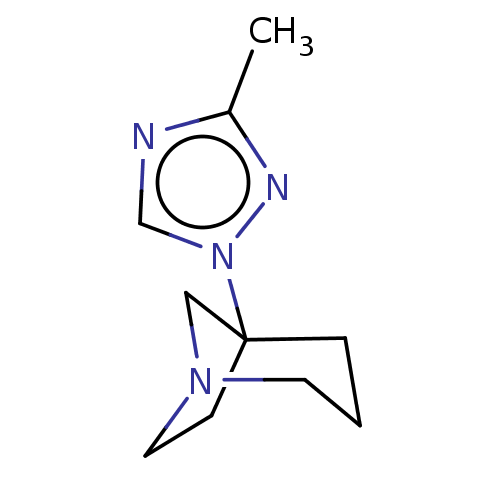
(CHEMBL311562)Show InChI InChI=1S/C10H16N4/c1-9-11-8-14(12-9)10-3-2-5-13(7-10)6-4-10/h8H,2-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229672

(CHEMBL309729)Show InChI InChI=1S/C9H15N5/c10-8-11-7-14(12-8)9-2-1-4-13(6-9)5-3-9/h7H,1-6H2,(H2,10,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50005677
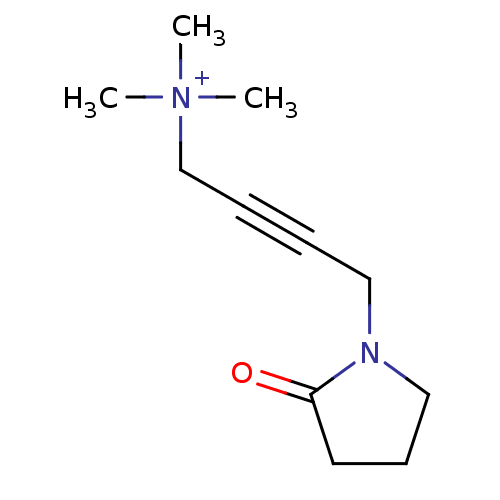
(CHEMBL23957 | OXO-M | Trimethyl-[4-(2-oxo-pyrrolid...)Show InChI InChI=1S/C11H19N2O/c1-13(2,3)10-5-4-8-12-9-6-7-11(12)14/h6-10H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex Muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50005677

(CHEMBL23957 | OXO-M | Trimethyl-[4-(2-oxo-pyrrolid...)Show InChI InChI=1S/C11H19N2O/c1-13(2,3)10-5-4-8-12-9-6-7-11(12)14/h6-10H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for its binding affinity to muscarinic acetylcholine receptor from rat cortical homogenates using [3H]OXO-M as radioliga... |
J Med Chem 35: 1280-90 (1992)
BindingDB Entry DOI: 10.7270/Q2G1632Q |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229526
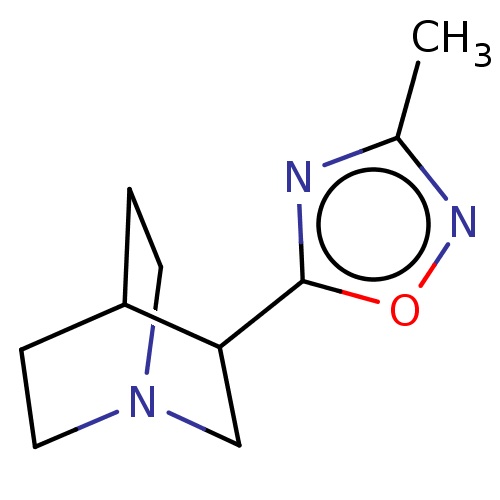
(CHEMBL279356)Show SMILES Cc1noc(n1)C1CN2CCC1CC2 |(5.41,-5.22,;4.1,-6,;4.34,-7.52,;2.94,-8.22,;1.87,-7.15,;2.56,-5.75,;.54,-7.93,;.54,-9.47,;-.8,-10.25,;-2.11,-9.47,;-2.11,-7.93,;-.8,-7.16,;-1.21,-8.65,;-.19,-8.97,)| Show InChI InChI=1S/C10H15N3O/c1-7-11-10(14-12-7)9-6-13-4-2-8(9)3-5-13/h8-9H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]OXO-M binding to Muscarinic receptor from rat cortical homogenates |
J Med Chem 35: 1280-90 (1992)
BindingDB Entry DOI: 10.7270/Q2G1632Q |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229693
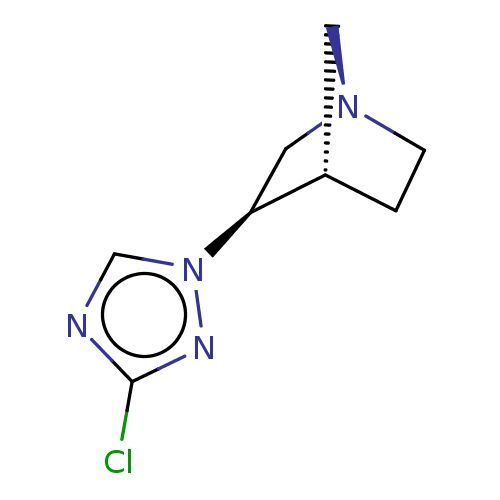
(CHEMBL312434)Show InChI InChI=1S/C8H11ClN4/c9-8-10-5-13(11-8)7-4-12-2-1-6(7)3-12/h5-7H,1-4H2/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex Muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229673

(CHEMBL311703)Show InChI InChI=1S/C9H15N5/c1-8-10-12-14(11-8)9-3-2-5-13(7-9)6-4-9/h2-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229526

(CHEMBL279356)Show SMILES Cc1noc(n1)C1CN2CCC1CC2 |(5.41,-5.22,;4.1,-6,;4.34,-7.52,;2.94,-8.22,;1.87,-7.15,;2.56,-5.75,;.54,-7.93,;.54,-9.47,;-.8,-10.25,;-2.11,-9.47,;-2.11,-7.93,;-.8,-7.16,;-1.21,-8.65,;-.19,-8.97,)| Show InChI InChI=1S/C10H15N3O/c1-7-11-10(14-12-7)9-6-13-4-2-8(9)3-5-13/h8-9H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for central muscarinic acetylcholine receptor affinity to displace [3H]oxotremorine-M from rat cerebral cortex; Valu... |
J Med Chem 34: 2726-35 (1991)
BindingDB Entry DOI: 10.7270/Q2BG2R7J |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229621
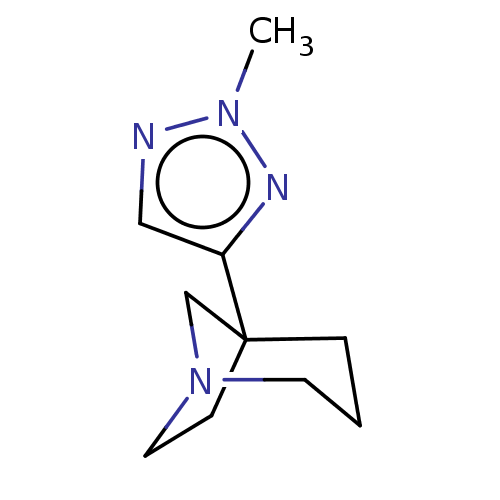
(CHEMBL311036)Show InChI InChI=1S/C10H16N4/c1-13-11-7-9(12-13)10-3-2-5-14(8-10)6-4-10/h7H,2-6,8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229524
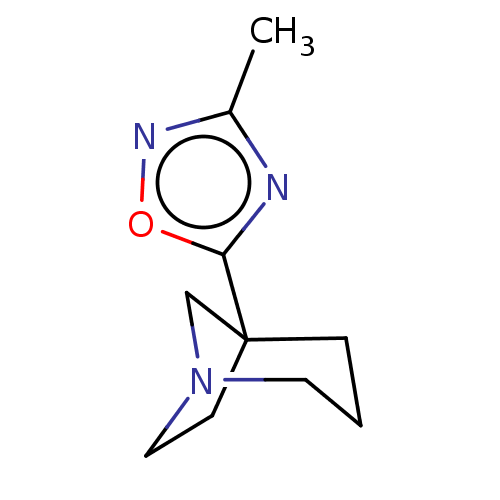
(CHEMBL329351)Show InChI InChI=1S/C10H15N3O/c1-8-11-9(14-12-8)10-3-2-5-13(7-10)6-4-10/h2-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for central Muscarinic acetylcholine receptor affinity to displace [3H]oxotremorine-M from rat cerebral cortex; Valu... |
J Med Chem 34: 2726-35 (1991)
BindingDB Entry DOI: 10.7270/Q2BG2R7J |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229683

(CHEMBL79020)Show InChI InChI=1S/C9H14N4/c1-12-10-4-9(11-12)8-6-13-3-2-7(8)5-13/h4,7-8H,2-3,5-6H2,1H3/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229698

(CHEMBL81492)Show InChI InChI=1S/C9H14N4/c1-7-10-6-13(11-7)9-5-12-3-2-8(9)4-12/h6,8-9H,2-5H2,1H3/t8-,9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50004665

((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...)Show InChI InChI=1S/C12H18N2O/c15-12-6-5-11-14(12)10-4-3-9-13-7-1-2-8-13/h1-2,5-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]OXO-M binding to Muscarinic receptor from rat cortical homogenates |
J Med Chem 35: 1280-90 (1992)
BindingDB Entry DOI: 10.7270/Q2G1632Q |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50004665

((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...)Show InChI InChI=1S/C12H18N2O/c15-12-6-5-11-14(12)10-4-3-9-13-7-1-2-8-13/h1-2,5-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229686
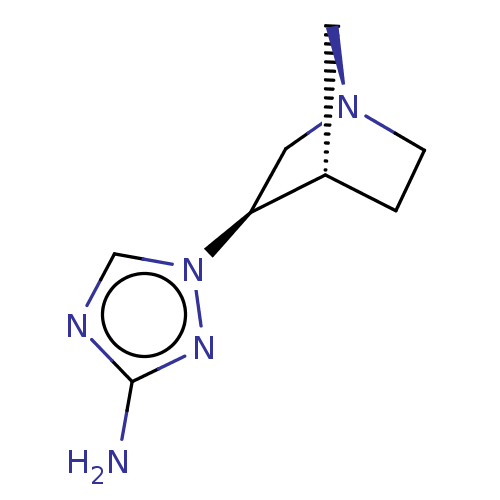
(CHEMBL311989)Show InChI InChI=1S/C8H13N5/c9-8-10-5-13(11-8)7-4-12-2-1-6(7)3-12/h5-7H,1-4H2,(H2,9,11)/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229530
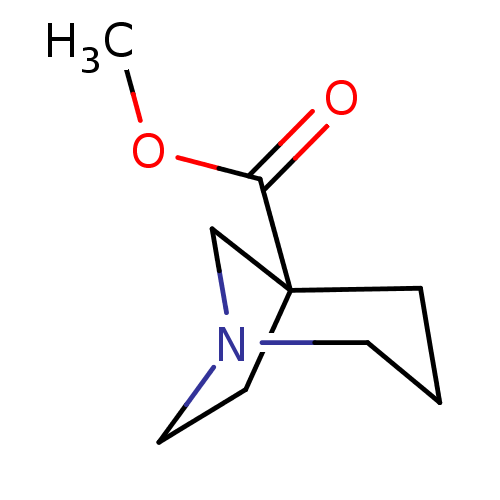
(CHEMBL329434)Show InChI InChI=1S/C9H15NO2/c1-12-8(11)9-3-2-5-10(7-9)6-4-9/h2-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for central muscarinic acetylcholine receptor affinity to displace [3H]oxotremorine-M from rat cerebral cortex; Valu... |
J Med Chem 34: 2726-35 (1991)
BindingDB Entry DOI: 10.7270/Q2BG2R7J |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229619
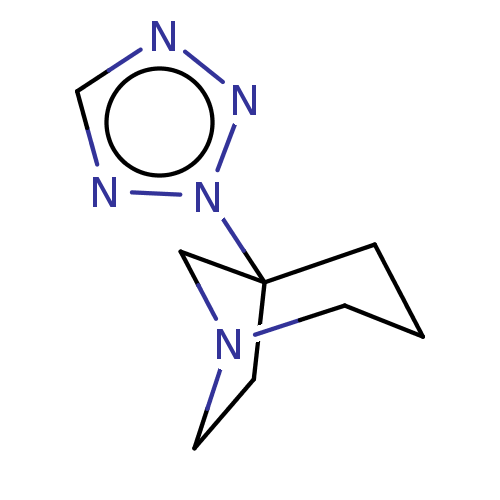
(CHEMBL442975)Show InChI InChI=1S/C8H13N5/c1-2-8(13-10-7-9-11-13)3-5-12(4-1)6-8/h7H,1-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229680
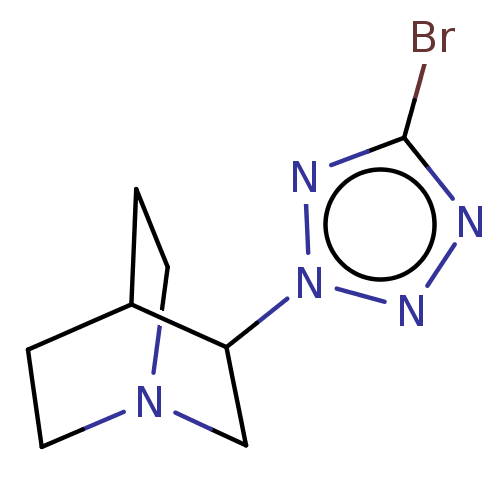
(CHEMBL312656)Show SMILES Brc1nnn(n1)C1CN2CCC1CC2 |(7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,)| Show InChI InChI=1S/C8H12BrN5/c9-8-10-12-14(11-8)7-5-13-3-1-6(7)2-4-13/h6-7H,1-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229614

(CHEMBL78964)Show SMILES Clc1nnn(n1)C1CN2CCC1CC2 |(7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,)| Show InChI InChI=1S/C8H12ClN5/c9-8-10-12-14(11-8)7-5-13-3-1-6(7)2-4-13/h6-7H,1-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229520
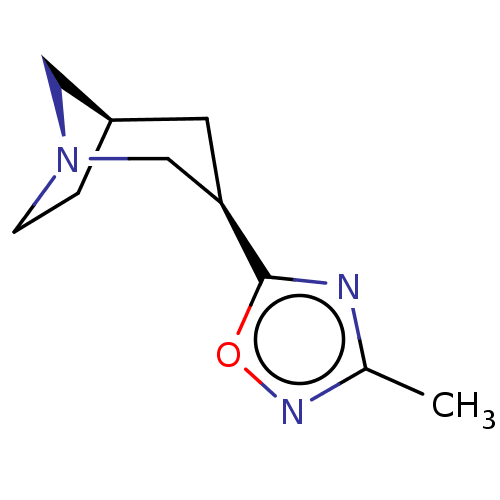
(CHEMBL93430)Show InChI InChI=1S/C10H15N3O/c1-7-11-10(14-12-7)9-4-8-2-3-13(5-8)6-9/h8-9H,2-6H2,1H3/t8-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for central muscarinic acetylcholine receptor affinity to displace [3H]oxotremorine-M from rat cerebral cortex; Valu... |
J Med Chem 34: 2726-35 (1991)
BindingDB Entry DOI: 10.7270/Q2BG2R7J |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229691
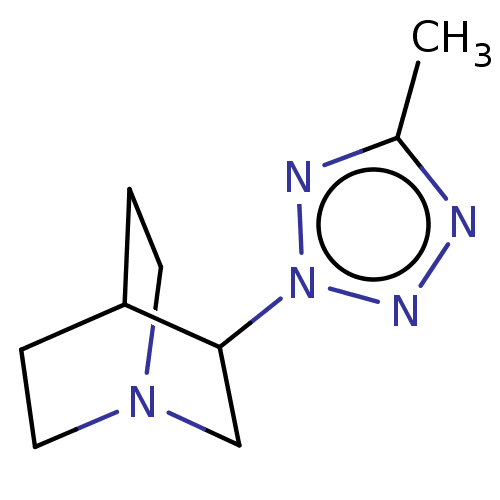
(CHEMBL23118)Show SMILES Cc1nnn(n1)C1CN2CCC1CC2 |(18.11,-12.96,;17.7,-14.41,;18.77,-15.8,;17.57,-17.13,;16.17,-16.52,;16.34,-14.95,;14.82,-17.27,;14.82,-18.82,;13.48,-19.48,;12.16,-18.82,;12.16,-17.27,;13.46,-16.5,;13.87,-17.98,;12.85,-18.33,)| Show InChI InChI=1S/C9H15N5/c1-7-10-12-14(11-7)9-6-13-4-2-8(9)3-5-13/h8-9H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for its binding affinity to muscarinic acetylcholine receptor from rat cortical homogenates using [3H]OXO-M as radioliga... |
J Med Chem 35: 1280-90 (1992)
BindingDB Entry DOI: 10.7270/Q2G1632Q |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229615
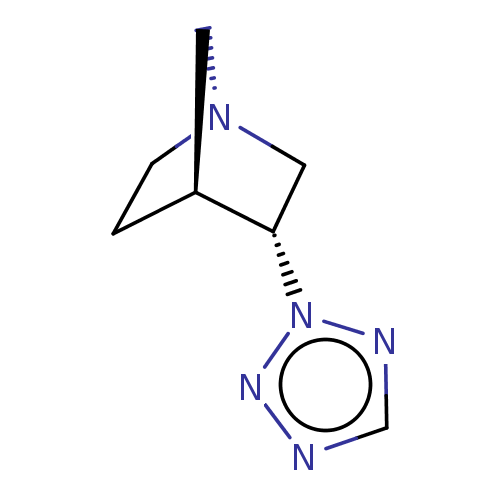
(CHEMBL81314)Show InChI InChI=1S/C7H11N5/c1-2-11-3-6(1)7(4-11)12-9-5-8-10-12/h5-7H,1-4H2/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229687
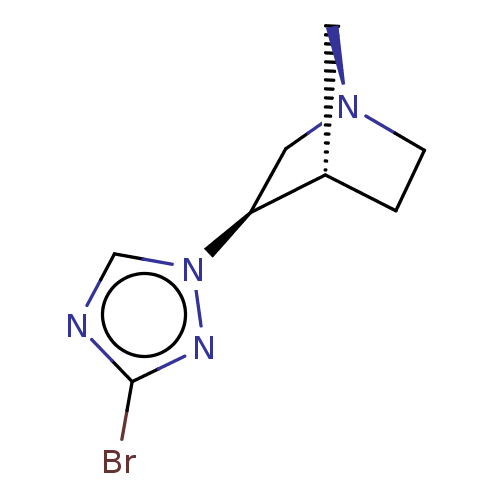
(CHEMBL308343)Show InChI InChI=1S/C8H11BrN4/c9-8-10-5-13(11-8)7-4-12-2-1-6(7)3-12/h5-7H,1-4H2/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex Muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229691

(CHEMBL23118)Show SMILES Cc1nnn(n1)C1CN2CCC1CC2 |(18.11,-12.96,;17.7,-14.41,;18.77,-15.8,;17.57,-17.13,;16.17,-16.52,;16.34,-14.95,;14.82,-17.27,;14.82,-18.82,;13.48,-19.48,;12.16,-18.82,;12.16,-17.27,;13.46,-16.5,;13.87,-17.98,;12.85,-18.33,)| Show InChI InChI=1S/C9H15N5/c1-7-10-12-14(11-7)9-6-13-4-2-8(9)3-5-13/h8-9H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229682
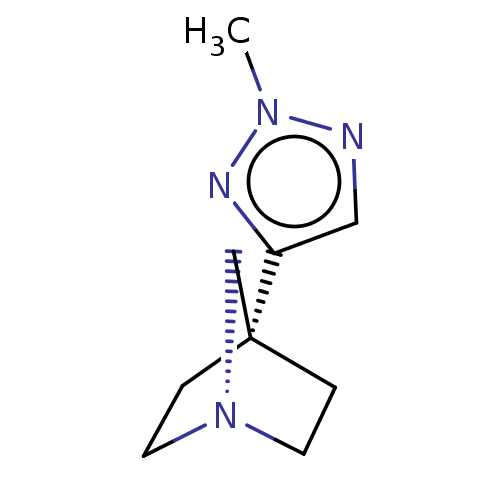
(CHEMBL79374)Show InChI InChI=1S/C9H14N4/c1-12-10-6-8(11-12)9-2-4-13(7-9)5-3-9/h6H,2-5,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229690

(CHEMBL311297)Show SMILES Nc1nnn(n1)C1CN2CCC1CC2 |(7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,)| Show InChI InChI=1S/C8H14N6/c9-8-10-12-14(11-8)7-5-13-3-1-6(7)2-4-13/h6-7H,1-5H2,(H2,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data