 Found 212 hits with Last Name = 'loughran' and Initial = 'hm'
Found 212 hits with Last Name = 'loughran' and Initial = 'hm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50280116
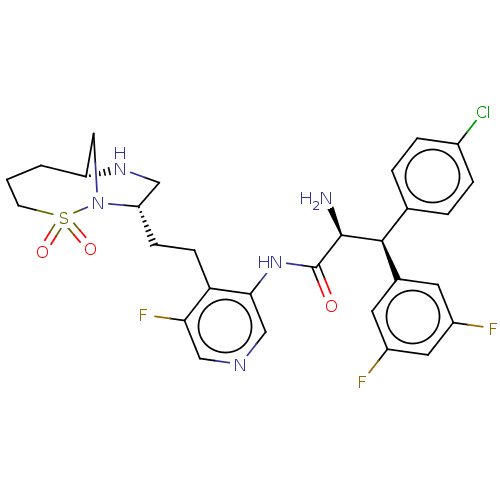
(CHEMBL4177355)Show SMILES [H][C@@]12CN([C@@H](CCc3c(F)cncc3NC(=O)[C@@H](N)[C@@H](c3ccc(Cl)cc3)c3cc(F)cc(F)c3)CN1)S(=O)(=O)CCC2 |r| Show InChI InChI=1S/C29H31ClF3N5O3S/c30-19-5-3-17(4-6-19)27(18-10-20(31)12-21(32)11-18)28(34)29(39)37-26-15-35-14-25(33)24(26)8-7-23-13-36-22-2-1-9-42(40,41)38(23)16-22/h3-6,10-12,14-15,22-23,27-28,36H,1-2,7-9,13,16,34H2,(H,37,39)/t22-,23+,27+,28+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli using Val-Ser-Gln-Asn-(beta-naphtyl)Ala-Pro-Ile-Val as substrate preincubated for 30 mins f... |
ACS Med Chem Lett 8: 1292-1297 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00386
BindingDB Entry DOI: 10.7270/Q2K35X57 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of GAR transformylase from Lactobacillus casei |
ACS Med Chem Lett 8: 1292-1297 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00386
BindingDB Entry DOI: 10.7270/Q2K35X57 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM13934

(Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...)Show SMILES COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1ccccn1)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C38H52N6O7/c1-37(2,3)31(41-35(48)50-7)33(46)40-29(22-25-14-10-9-11-15-25)30(45)24-44(43-34(47)32(38(4,5)6)42-36(49)51-8)23-26-17-19-27(20-18-26)28-16-12-13-21-39-28/h9-21,29-32,45H,22-24H2,1-8H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)/t29-,30-,31+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli using Val-Ser-Gln-Asn-(beta-naphtyl)Ala-Pro-Ile-Val as substrate preincubated for 30 mins f... |
ACS Med Chem Lett 8: 1292-1297 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00386
BindingDB Entry DOI: 10.7270/Q2K35X57 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50190623
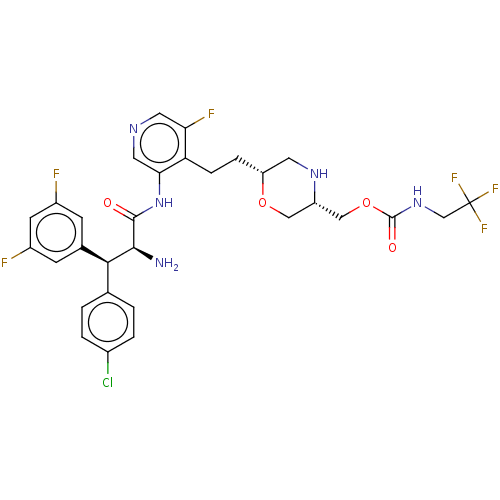
(CHEMBL3828743)Show SMILES N[C@@H]([C@@H](c1ccc(Cl)cc1)c1cc(F)cc(F)c1)C(=O)Nc1cncc(F)c1CC[C@@H]1CN[C@H](COC(=O)NCC(F)(F)F)CO1 |r| Show InChI InChI=1S/C30H30ClF6N5O4/c31-18-3-1-16(2-4-18)26(17-7-19(32)9-20(33)8-17)27(38)28(43)42-25-12-39-11-24(34)23(25)6-5-22-10-40-21(13-45-22)14-46-29(44)41-15-30(35,36)37/h1-4,7-9,11-12,21-22,26-27,40H,5-6,10,13-15,38H2,(H,41,44)(H,42,43)/t21-,22+,26-,27-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease expressed in Escherichia coli using Val-Ser-Gln-Asn-(beta-naphtyl)Ala-Pro-Ile-Val as substrate preincubated for 30 mins f... |
ACS Med Chem Lett 8: 1292-1297 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00386
BindingDB Entry DOI: 10.7270/Q2K35X57 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406127
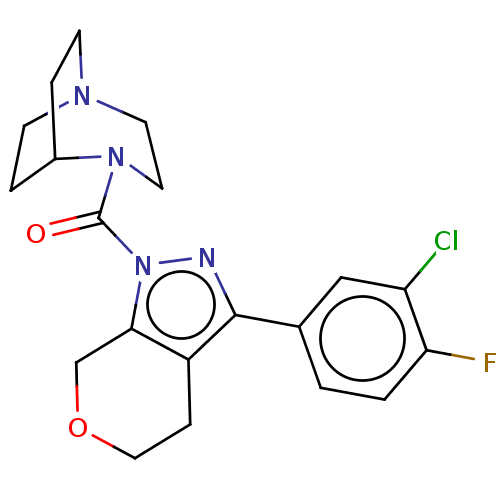
(CHEMBL5290844)Show SMILES Cc1cc(C)c(CCCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C22H28FO5P/c1-14-9-15(2)19(20(10-14)17-6-7-21(23)16(3)11-17)5-4-8-29(27,28)13-18(24)12-22(25)26/h6-7,9-11,27-29H,4-5,8,12-13H2,1-3H3,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406126

(CHEMBL5291288)Show SMILES CC(C)c1c(CCP(O)(O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 Show InChI InChI=1S/C23H27FNO5P/c1-15(2)23-19-5-3-4-6-20(19)25(17-9-7-16(24)8-10-17)21(23)11-12-31(29,30)14-18(26)13-22(27)28/h3-10,15,29-31H,11-14H2,1-2H3,(H,27,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406123
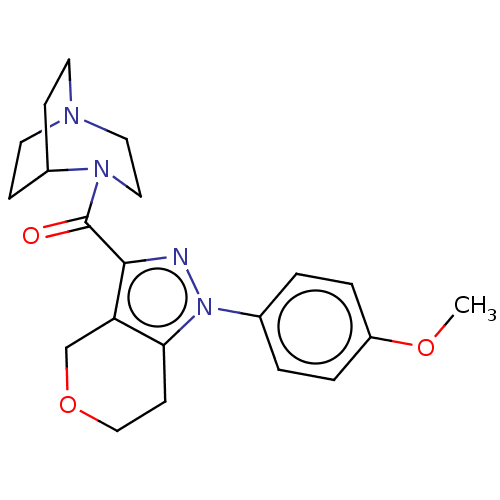
(CHEMBL5277419)Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1CCP(O)(O)CC(=O)CC(O)=O Show InChI InChI=1S/C23H30FO5P/c1-14(2)20-9-15(3)10-21(17-5-6-22(24)16(4)11-17)19(20)7-8-30(28,29)13-18(25)12-23(26)27/h5-6,9-11,14,28-30H,7-8,12-13H2,1-4H3,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50593123
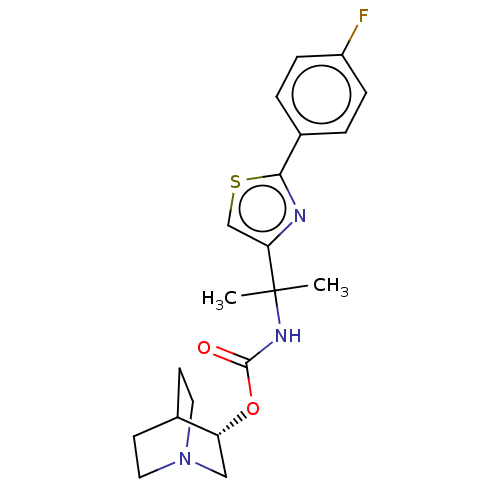
(GENZ-682452 | GZ-402671 | GZ/SAR402671 | GZ402671 ...)Show SMILES CC(C)(NC(=O)O[C@@H]1CN2CCC1CC2)c1csc(n1)-c1ccc(F)cc1 |r,wU:7.6,(-6.59,.2,;-5.85,-1.18,;-5.38,.09,;-4.52,-1.95,;-3.19,-1.18,;-3.19,.36,;-1.85,-1.95,;-.52,-1.18,;.81,-1.95,;2.15,-1.18,;2.15,.36,;.81,1.13,;-.52,.36,;.5,.07,;1.39,-.44,;-7.1,-2.08,;-7.1,-3.62,;-8.57,-4.1,;-9.47,-2.85,;-8.57,-1.6,;-11.01,-2.85,;-11.78,-1.52,;-13.32,-1.52,;-14.09,-2.85,;-15.63,-2.85,;-13.32,-4.18,;-11.78,-4.18,)| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against bovine cathepsin D |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Mus musculus) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Mus musculus) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406132

(CHEMBL5273314)Show SMILES CC(C)n1c(C=CP(O)(=O)CC(=O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)25-20-6-4-3-5-19(20)23(16-7-9-17(24)10-8-16)21(25)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406133

(CHEMBL5288633)Show SMILES COP(=O)(C[C@@H](O)CC(O)=O)NCc1c(C)cc(C)cc1-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H27FNO5P/c1-13-7-14(2)19(18(8-13)16-5-6-20(22)15(3)9-16)11-23-29(27,28-4)12-17(24)10-21(25)26/h5-9,17,24H,10-12H2,1-4H3,(H,23,27)(H,25,26)/t17-,29?/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406137

(CHEMBL5286066)Show InChI InChI=1S/C21H27N3O/c1-18-7-5-6-10-20(18)21(25)22-11-12-23-13-15-24(16-14-23)17-19-8-3-2-4-9-19/h2-10H,11-17H2,1H3,(H,22,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406136

(CHEMBL5278676)Show SMILES CCc1c(C=CP(O)(=O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 |w:5.5| Show InChI InChI=1S/C22H21FNO5P/c1-2-18-19-5-3-4-6-20(19)24(16-9-7-15(23)8-10-16)21(18)11-12-30(28,29)14-17(25)13-22(26)27/h3-12H,2,13-14H2,1H3,(H,26,27)(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against aspartic proteinases pepsin from porcine |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406138
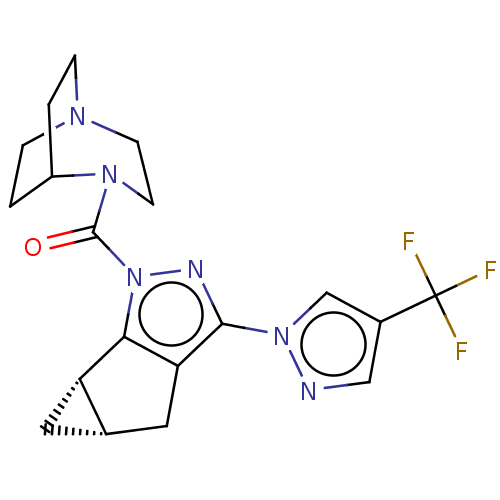
(CHEMBL5286071)Show SMILES [O-][N+](=O)c1ccccc1NC(=O)CCN1CCN2Cc3[nH]c4ccccc4c3CC2C1 Show InChI InChI=1S/C23H25N5O3/c29-23(25-20-7-3-4-8-22(20)28(30)31)9-10-26-11-12-27-15-21-18(13-16(27)14-26)17-5-1-2-6-19(17)24-21/h1-8,16,24H,9-15H2,(H,25,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406131
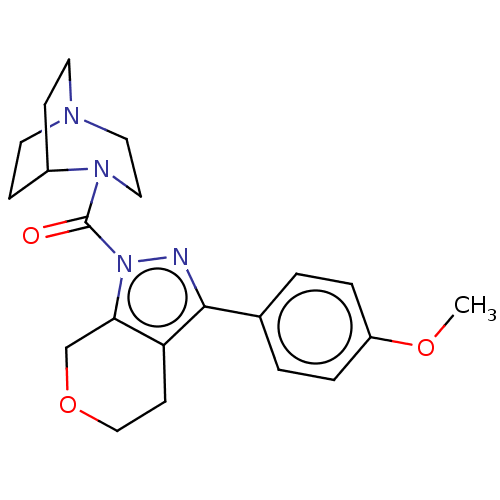
(CHEMBL5278405)Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1C=CP(O)(=O)CC(=O)CC(O)=O |w:19.21| Show InChI InChI=1S/C23H26FO5P/c1-14(2)20-9-15(3)10-21(17-5-6-22(24)16(4)11-17)19(20)7-8-30(28,29)13-18(25)12-23(26)27/h5-11,14H,12-13H2,1-4H3,(H,26,27)(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406135

(CHEMBL5278640)Show SMILES CC(C)c1c(C=CP(O)(=O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)23-19-5-3-4-6-20(19)25(17-9-7-16(24)8-10-17)21(23)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50593123

(GENZ-682452 | GZ-402671 | GZ/SAR402671 | GZ402671 ...)Show SMILES CC(C)(NC(=O)O[C@@H]1CN2CCC1CC2)c1csc(n1)-c1ccc(F)cc1 |r,wU:7.6,(-6.59,.2,;-5.85,-1.18,;-5.38,.09,;-4.52,-1.95,;-3.19,-1.18,;-3.19,.36,;-1.85,-1.95,;-.52,-1.18,;.81,-1.95,;2.15,-1.18,;2.15,.36,;.81,1.13,;-.52,.36,;.5,.07,;1.39,-.44,;-7.1,-2.08,;-7.1,-3.62,;-8.57,-4.1,;-9.47,-2.85,;-8.57,-1.6,;-11.01,-2.85,;-11.78,-1.52,;-13.32,-1.52,;-14.09,-2.85,;-15.63,-2.85,;-13.32,-4.18,;-11.78,-4.18,)| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482918
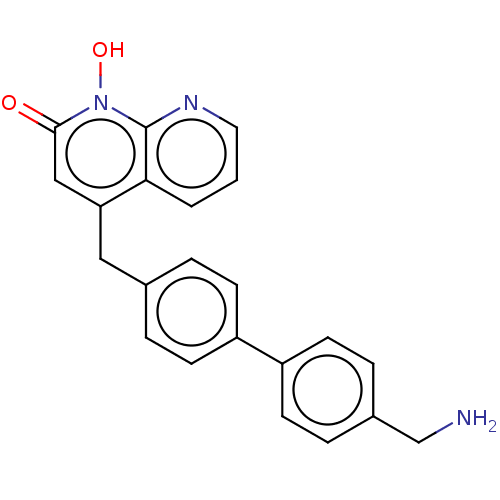
(CHEMBL1270611)Show SMILES NCc1ccc(cc1)-c1ccc(Cc2cc(=O)n(O)c3ncccc23)cc1 Show InChI InChI=1S/C22H19N3O2/c23-14-16-5-9-18(10-6-16)17-7-3-15(4-8-17)12-19-13-21(26)25(27)22-20(19)2-1-11-24-22/h1-11,13,27H,12,14,23H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase RNasH activity |
Bioorg Med Chem Lett 20: 6754-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.135
BindingDB Entry DOI: 10.7270/Q21N8405 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406119

(CHEMBL5273405)Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1COP(O)(=O)C[C@@H](O)CC(O)=O Show InChI InChI=1S/C22H28FO6P/c1-13(2)18-7-14(3)8-19(16-5-6-21(23)15(4)9-16)20(18)11-29-30(27,28)12-17(24)10-22(25)26/h5-9,13,17,24H,10-12H2,1-4H3,(H,25,26)(H,27,28)/t17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482921
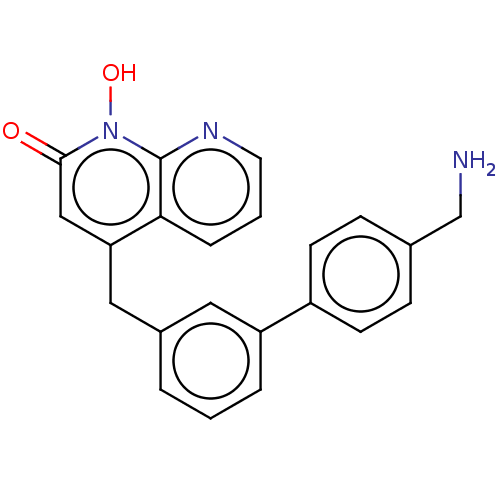
(CHEMBL1270511)Show SMILES NCc1ccc(cc1)-c1cccc(Cc2cc(=O)n(O)c3ncccc23)c1 Show InChI InChI=1S/C22H19N3O2/c23-14-15-6-8-17(9-7-15)18-4-1-3-16(11-18)12-19-13-21(26)25(27)22-20(19)5-2-10-24-22/h1-11,13,27H,12,14,23H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase RNasH activity |
Bioorg Med Chem Lett 20: 6754-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.135
BindingDB Entry DOI: 10.7270/Q21N8405 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM549569
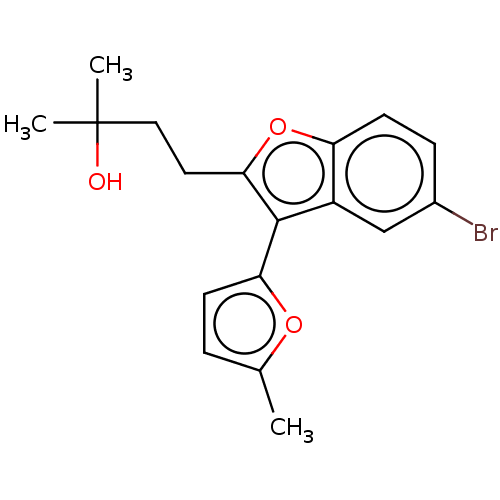
(US11306075, Ex. 15) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tau target (equal mixture of each construct at 300 nM) was prepared in buffer (Tris-HCl pH 7.4) and was incubated in 96-well plates at room temperatu... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WS8XFN |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM549563
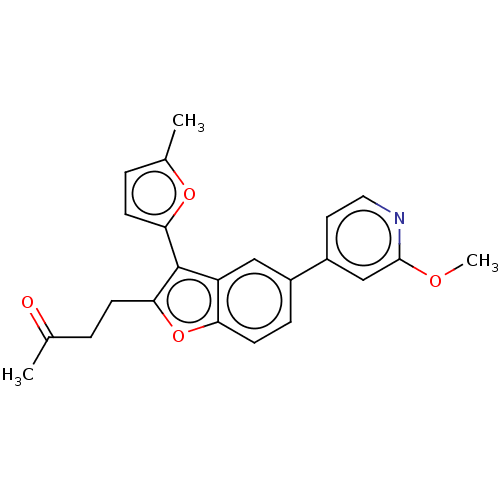
(US11306075, Ex. 7)Show SMILES COc1cc(ccn1)-c1ccc2oc(CCC(C)=O)c(-c3ccc(C)o3)c2c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tau target (equal mixture of each construct at 300 nM) was prepared in buffer (Tris-HCl pH 7.4) and was incubated in 96-well plates at room temperatu... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WS8XFN |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482925
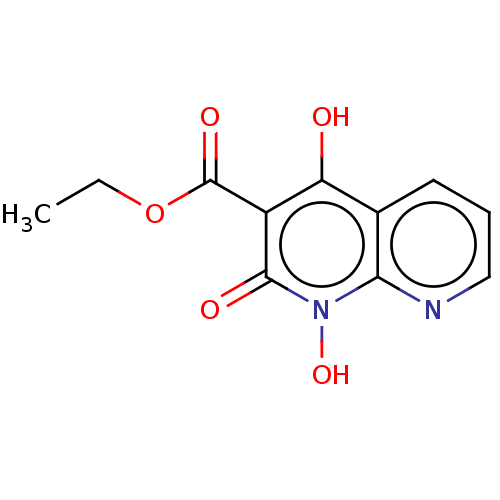
(CHEMBL1269455)Show InChI InChI=1S/C11H10N2O5/c1-2-18-11(16)7-8(14)6-4-3-5-12-9(6)13(17)10(7)15/h3-5,14,17H,2H2,1H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase RNasH activity |
Bioorg Med Chem Lett 20: 6754-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.135
BindingDB Entry DOI: 10.7270/Q21N8405 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482919
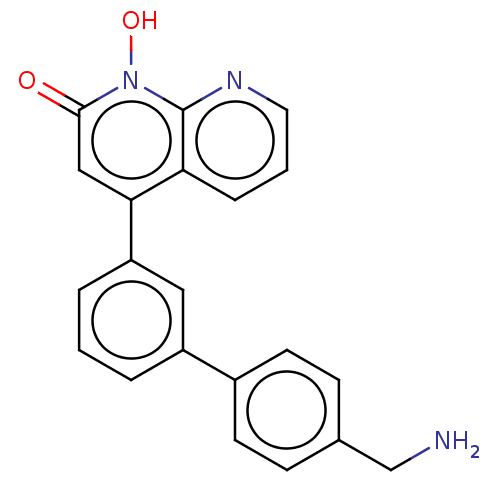
(CHEMBL1270510)Show SMILES NCc1ccc(cc1)-c1cccc(c1)-c1cc(=O)n(O)c2ncccc12 Show InChI InChI=1S/C21H17N3O2/c22-13-14-6-8-15(9-7-14)16-3-1-4-17(11-16)19-12-20(25)24(26)21-18(19)5-2-10-23-21/h1-12,26H,13,22H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase RNasH activity |
Bioorg Med Chem Lett 20: 6754-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.135
BindingDB Entry DOI: 10.7270/Q21N8405 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482920
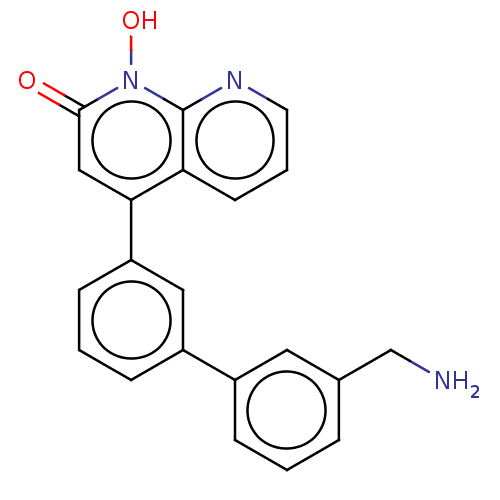
(CHEMBL1270417)Show SMILES NCc1cccc(c1)-c1cccc(c1)-c1cc(=O)n(O)c2ncccc12 Show InChI InChI=1S/C21H17N3O2/c22-13-14-4-1-5-15(10-14)16-6-2-7-17(11-16)19-12-20(25)24(26)21-18(19)8-3-9-23-21/h1-12,26H,13,22H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase RNasH activity |
Bioorg Med Chem Lett 20: 6754-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.135
BindingDB Entry DOI: 10.7270/Q21N8405 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406128

(CHEMBL5270537)Show SMILES OC(=O)CC(=O)CP(O)(=O)CCC1=C(c2ccccc2C11CCCC1)c1ccc(F)cc1 |t:12| Show InChI InChI=1S/C25H26FO5P/c26-18-9-7-17(8-10-18)24-20-5-1-2-6-21(20)25(12-3-4-13-25)22(24)11-14-32(30,31)16-19(27)15-23(28)29/h1-2,5-10H,3-4,11-16H2,(H,28,29)(H,30,31) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406131

(CHEMBL5278405)Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1C=CP(O)(=O)CC(=O)CC(O)=O |w:19.21| Show InChI InChI=1S/C23H26FO5P/c1-14(2)20-9-15(3)10-21(17-5-6-22(24)16(4)11-17)19(20)7-8-30(28,29)13-18(25)12-23(26)27/h5-11,14H,12-13H2,1-4H3,(H,26,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 338 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
ETA receptor antagonist activity was measured by inhibition of ET-1 induced vasoconstriction in isolated porcine coronary artery |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406135

(CHEMBL5278640)Show SMILES CC(C)c1c(C=CP(O)(=O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)23-19-5-3-4-6-20(19)25(17-9-7-16(24)8-10-17)21(23)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 585 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482922
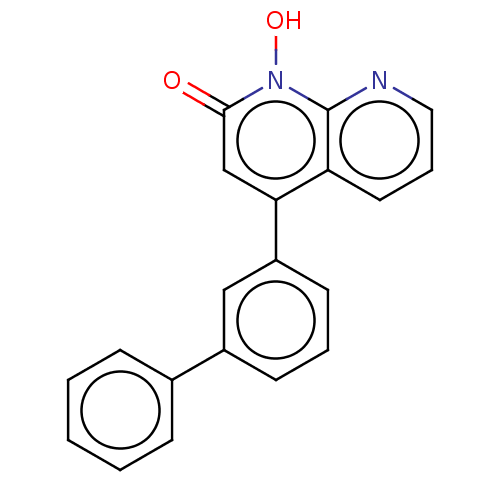
(CHEMBL1270317)Show InChI InChI=1S/C20H14N2O2/c23-19-13-18(17-10-5-11-21-20(17)22(19)24)16-9-4-8-15(12-16)14-6-2-1-3-7-14/h1-13,24H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase RNasH activity |
Bioorg Med Chem Lett 20: 6754-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.135
BindingDB Entry DOI: 10.7270/Q21N8405 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482924
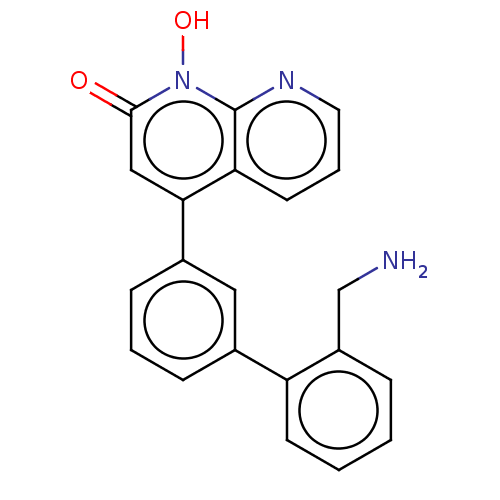
(CHEMBL1270416)Show SMILES NCc1ccccc1-c1cccc(c1)-c1cc(=O)n(O)c2ncccc12 Show InChI InChI=1S/C21H17N3O2/c22-13-16-5-1-2-8-17(16)14-6-3-7-15(11-14)19-12-20(25)24(26)21-18(19)9-4-10-23-21/h1-12,26H,13,22H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase RNasH activity |
Bioorg Med Chem Lett 20: 6754-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.135
BindingDB Entry DOI: 10.7270/Q21N8405 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406118
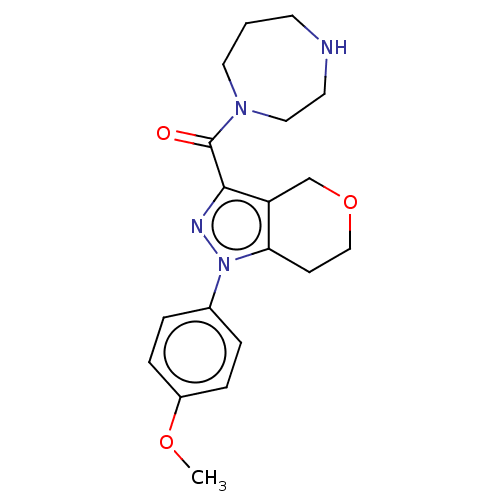
(CHEMBL5281181)Show SMILES CC(C)n1c(CCP(O)(O)CC(=O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 Show InChI InChI=1S/C23H27FNO5P/c1-15(2)25-20-6-4-3-5-19(20)23(16-7-9-17(24)10-8-16)21(25)11-12-31(29,30)14-18(26)13-22(27)28/h3-10,15,29-31H,11-14H2,1-2H3,(H,27,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM33410
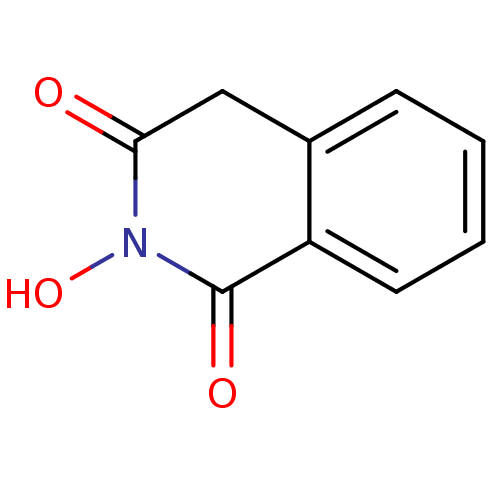
(CHEMBL16755 | N-hydroxyisoquinolinedione, 2)Show InChI InChI=1S/C9H7NO3/c11-8-5-6-3-1-2-4-7(6)9(12)10(8)13/h1-4,13H,5H2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase RNasH activity |
Bioorg Med Chem Lett 20: 6754-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.135
BindingDB Entry DOI: 10.7270/Q21N8405 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406128

(CHEMBL5270537)Show SMILES OC(=O)CC(=O)CP(O)(=O)CCC1=C(c2ccccc2C11CCCC1)c1ccc(F)cc1 |t:12| Show InChI InChI=1S/C25H26FO5P/c26-18-9-7-17(8-10-18)24-20-5-1-2-6-21(20)25(12-3-4-13-25)22(24)11-14-32(30,31)16-19(27)15-23(28)29/h1-2,5-10H,3-4,11-16H2,(H,28,29)(H,30,31) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM549574
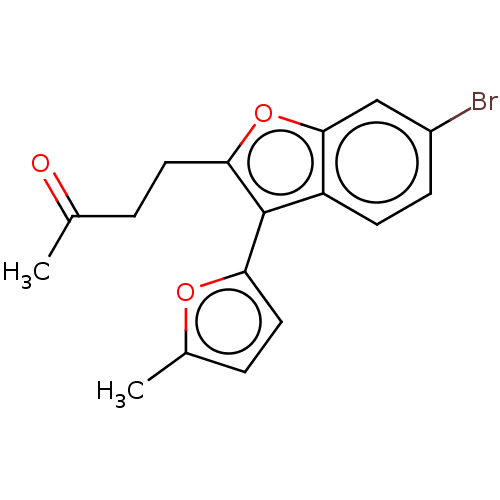
(US11306075, Ex. 21) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tau target (equal mixture of each construct at 300 nM) was prepared in buffer (Tris-HCl pH 7.4) and was incubated in 96-well plates at room temperatu... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WS8XFN |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM549558
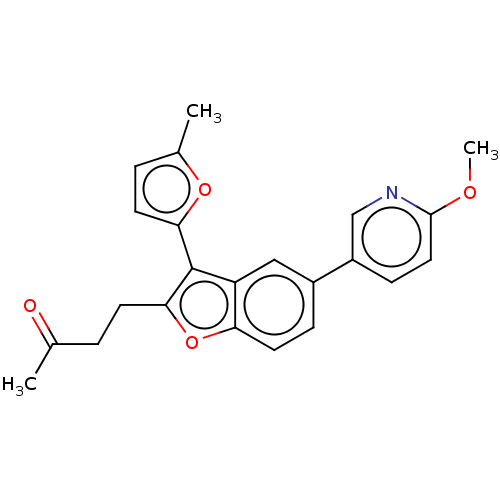
(US11306075, Ex. 2)Show SMILES COc1ccc(cn1)-c1ccc2oc(CCC(C)=O)c(-c3ccc(C)o3)c2c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tau target (equal mixture of each construct at 300 nM) was prepared in buffer (Tris-HCl pH 7.4) and was incubated in 96-well plates at room temperatu... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WS8XFN |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM549564
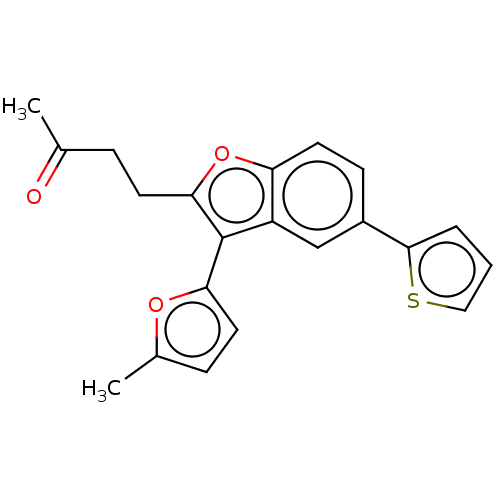
(US11306075, Ex. 8) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tau target (equal mixture of each construct at 300 nM) was prepared in buffer (Tris-HCl pH 7.4) and was incubated in 96-well plates at room temperatu... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WS8XFN |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM549560
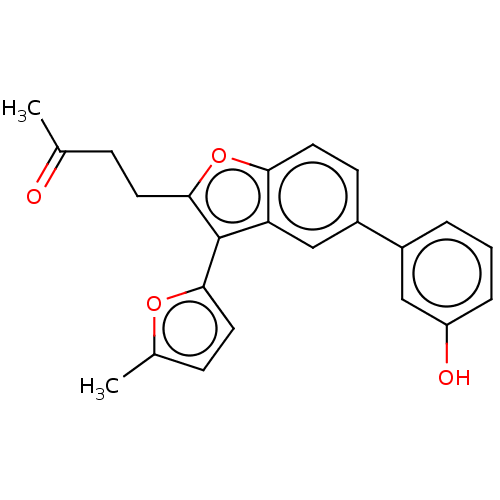
(US11306075, Ex. 4)Show SMILES CC(=O)CCc1oc2ccc(cc2c1-c1ccc(C)o1)-c1cccc(O)c1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tau target (equal mixture of each construct at 300 nM) was prepared in buffer (Tris-HCl pH 7.4) and was incubated in 96-well plates at room temperatu... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WS8XFN |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50482923
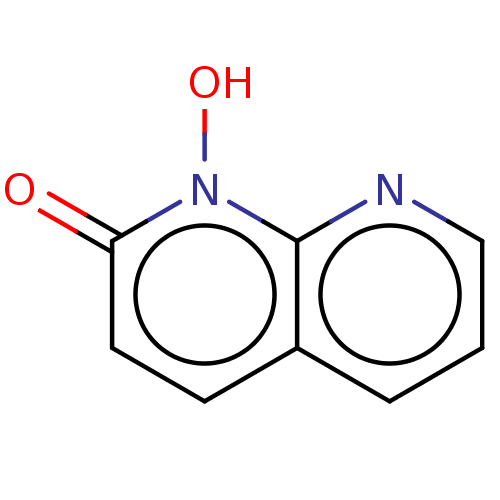
(CHEMBL1269478)Show InChI InChI=1S/C8H6N2O2/c11-7-4-3-6-2-1-5-9-8(6)10(7)12/h1-5,12H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 reverse transcriptase RNasH activity |
Bioorg Med Chem Lett 20: 6754-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.135
BindingDB Entry DOI: 10.7270/Q21N8405 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM549576

(US11306075, Ex. 23)Show SMILES COc1cc(ccn1)-c1ccc2c(c(CCC(C)=O)oc2c1)-c1ccc(C)o1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tau target (equal mixture of each construct at 300 nM) was prepared in buffer (Tris-HCl pH 7.4) and was incubated in 96-well plates at room temperatu... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WS8XFN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406132

(CHEMBL5273314)Show SMILES CC(C)n1c(C=CP(O)(=O)CC(=O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)25-20-6-4-3-5-19(20)23(16-7-9-17(24)10-8-16)21(25)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Isoform Tau-F of Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM576627
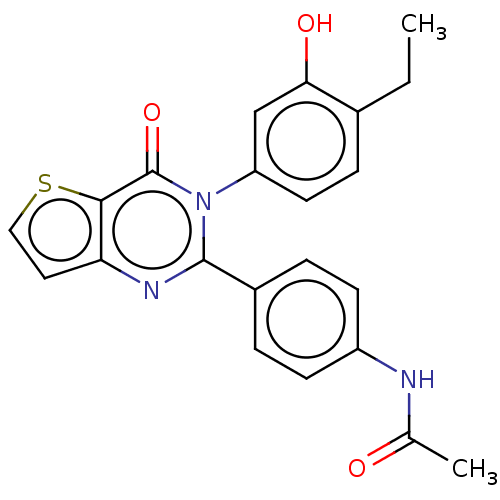
(US11472776, Example 16)Show SMILES CCc1ccc(cc1O)-n1c(nc2ccsc2c1=O)-c1ccc(NC(C)=O)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Confirmatory Assay (CONFA) used as a secondary screen for confirming the mechanism of action of compounds identified as hits using the ALA assay. The... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZW1Q5K |
More data for this
Ligand-Target Pair | |
Isoform Tau-F of Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM576467

(US11472776, Example 1) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
AlphaLisa Assay (ALA) was used as a primary screen for identifying compounds that inhibit tau oligomer formation. It is a bead based assay that only ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZW1Q5K |
More data for this
Ligand-Target Pair | |
Isoform Tau-F of Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM576631
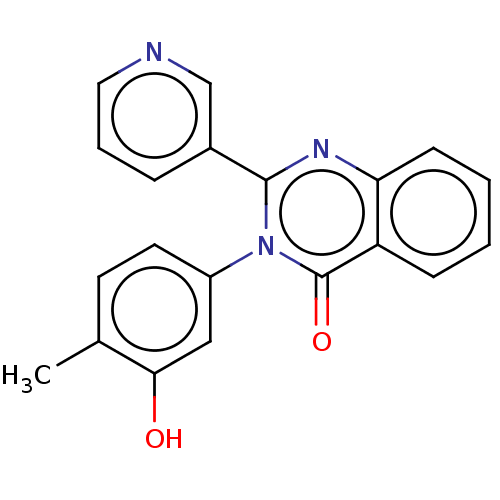
(US11472776, Example 20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
AlphaLisa Assay (ALA) was used as a primary screen for identifying compounds that inhibit tau oligomer formation. It is a bead based assay that only ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZW1Q5K |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM549570
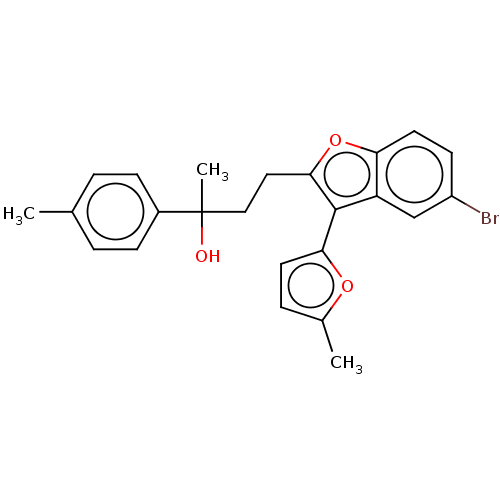
(US11306075, Ex. 16)Show SMILES Cc1ccc(o1)-c1c(CCC(C)(O)c2ccc(C)cc2)oc2ccc(Br)cc12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tau target (equal mixture of each construct at 300 nM) was prepared in buffer (Tris-HCl pH 7.4) and was incubated in 96-well plates at room temperatu... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WS8XFN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data