

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50569443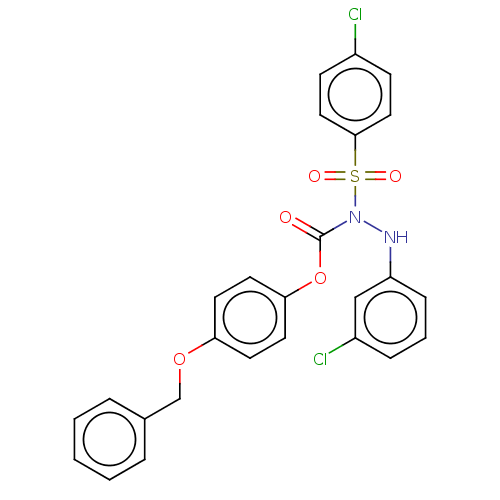 (CHEMBL4851179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128920 BindingDB Entry DOI: 10.7270/Q23B6438 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50314723 (CHEMBL1090041 | endo-(S)-N-(4-fluorobenzyl)-4-(3-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in CHO cells assessed as inhibition of RANTES-induced [32S]GTPgammaS binding | Bioorg Med Chem Lett 20: 2219-23 (2010) Article DOI: 10.1016/j.bmcl.2010.02.023 BindingDB Entry DOI: 10.7270/Q2C53M0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50314722 (CHEMBL1093331 | endo-(S)-methyl 4-(3-(2-methyl-3H-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor expressed in CHO cells assessed as inhibition of RANTES-induced [32S]GTPgammaS binding | Bioorg Med Chem Lett 20: 2219-23 (2010) Article DOI: 10.1016/j.bmcl.2010.02.023 BindingDB Entry DOI: 10.7270/Q2C53M0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321725 (CHEMBL1170924 | endo-N-((S)-1-(4-(Trifluoromethyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-stimulated GTPgammaS binding by scintillation proximity assay | Eur J Med Chem 45: 2827-40 (2010) Article DOI: 10.1016/j.ejmech.2010.03.003 BindingDB Entry DOI: 10.7270/Q2FB5341 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50359989 (CHEMBL1927943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reagent method | J Nat Prod 74: 2489-96 (2011) Article DOI: 10.1021/np100874f BindingDB Entry DOI: 10.7270/Q2ST7Q8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479075 (CHEMBL492770) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479066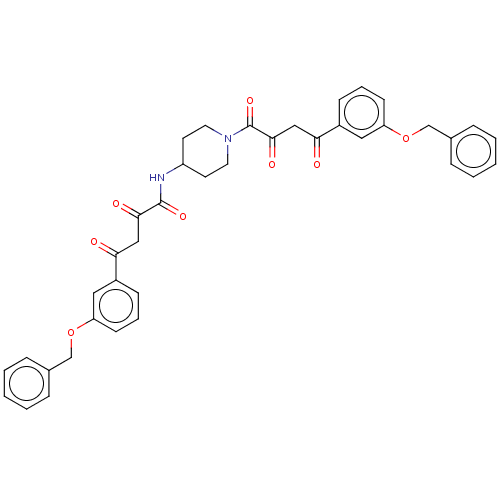 (CHEMBL454190) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479081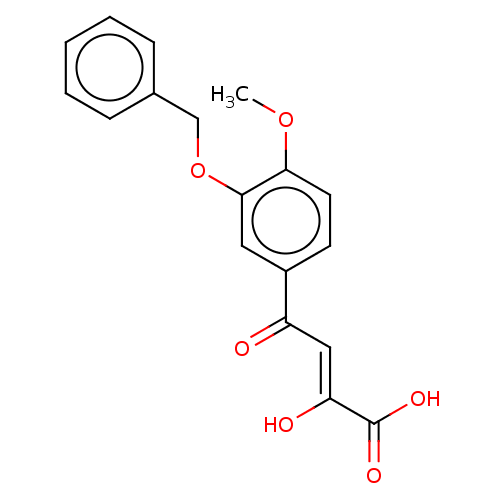 (CHEMBL467149) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479058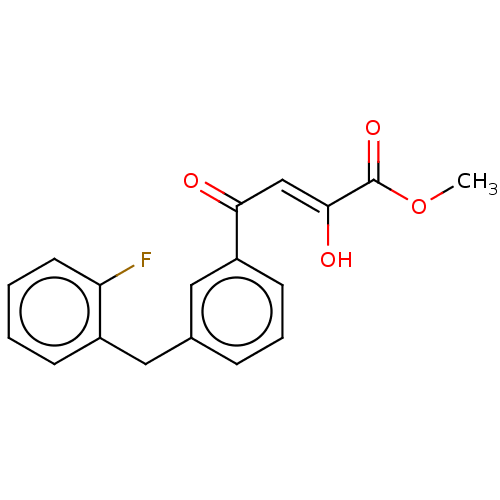 (CHEMBL467380) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479065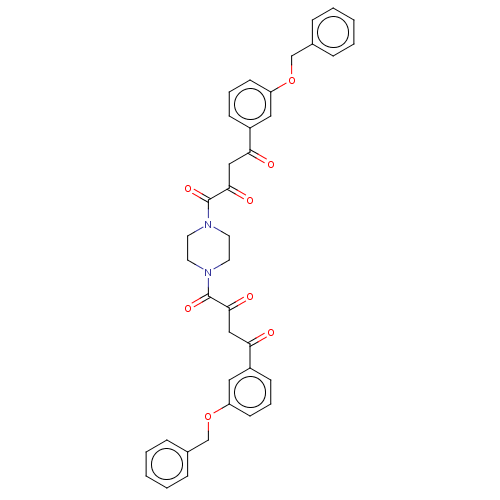 (CHEMBL443890) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479064 (CHEMBL452223) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479057 (CHEMBL444974) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479067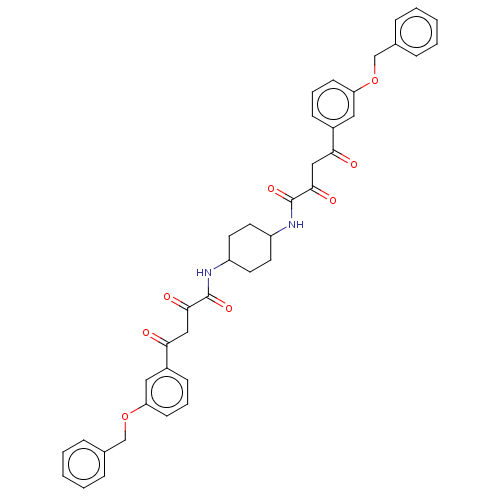 (CHEMBL452876) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479072 (CHEMBL467361) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50509618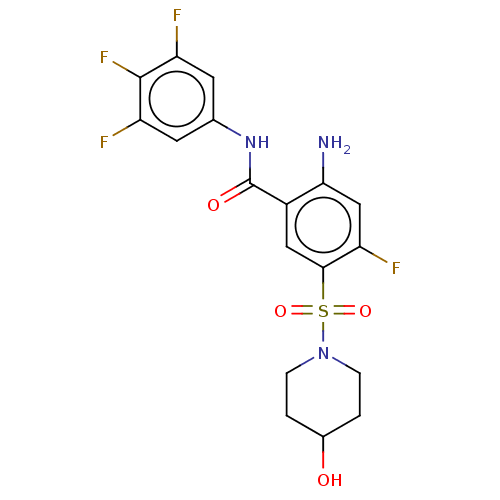 (CHEMBL4463663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in human HEK293 cells by patch clamp assay | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50353025 (CHEMBL1821987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321724 (CHEMBL1170923 | endo-4-Fluoro-N-((S)-3-(3-(2-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-stimulated GTPgammaS binding by scintillation proximity assay | Eur J Med Chem 45: 2827-40 (2010) Article DOI: 10.1016/j.ejmech.2010.03.003 BindingDB Entry DOI: 10.7270/Q2FB5341 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321723 (CHEMBL1170925 | N-((S)-3-(4-(4-(N-benzyl-N-isoprop...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-stimulated GTPgammaS binding by scintillation proximity assay | Eur J Med Chem 45: 2827-40 (2010) Article DOI: 10.1016/j.ejmech.2010.03.003 BindingDB Entry DOI: 10.7270/Q2FB5341 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321722 (CHEMBL1169745 | N-((S)-1-(4-(trifluoromethyl)pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-stimulated GTPgammaS binding by scintillation proximity assay | Eur J Med Chem 45: 2827-40 (2010) Article DOI: 10.1016/j.ejmech.2010.03.003 BindingDB Entry DOI: 10.7270/Q2FB5341 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321721 (CHEMBL1170529 | Methyl-4-benzyl-1-((S)-3-(cyclobut...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-stimulated GTPgammaS binding by scintillation proximity assay | Eur J Med Chem 45: 2827-40 (2010) Article DOI: 10.1016/j.ejmech.2010.03.003 BindingDB Entry DOI: 10.7270/Q2FB5341 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321720 (CHEMBL1170738 | N-((S)-3-(4-benzyl-4-(hydroxymethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-stimulated GTPgammaS binding by scintillation proximity assay | Eur J Med Chem 45: 2827-40 (2010) Article DOI: 10.1016/j.ejmech.2010.03.003 BindingDB Entry DOI: 10.7270/Q2FB5341 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50321719 (CHEMBL1172070 | Methyl-4-benzyl-1-((S)-3-(cyclobut...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Antagonist activity at CCR5 expressed in CHO cells assessed as inhibition of RANTES-stimulated GTPgammaS binding by scintillation proximity assay | Eur J Med Chem 45: 2827-40 (2010) Article DOI: 10.1016/j.ejmech.2010.03.003 BindingDB Entry DOI: 10.7270/Q2FB5341 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50359990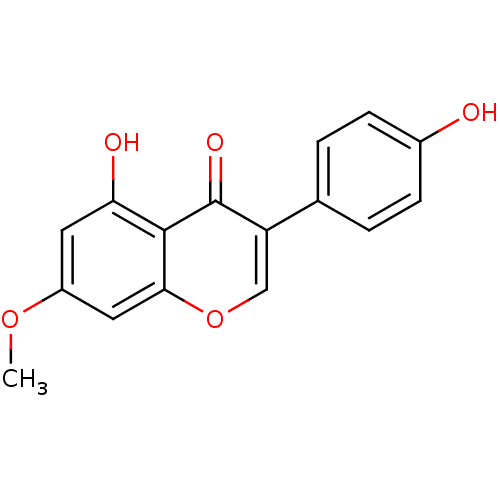 (PRUNETIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reagent method | J Nat Prod 74: 2489-96 (2011) Article DOI: 10.1021/np100874f BindingDB Entry DOI: 10.7270/Q2ST7Q8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50509618 (CHEMBL4463663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human ERG by competitive fluorescence polarization assay | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479048 (CHEMBL476930) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479068 (CHEMBL511423) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479076 (CHEMBL452111) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50353027 (CHEMBL1821990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479056 (CHEMBL448880) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50509618 (CHEMBL4463663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 using tolubutamide as substrate by LC/MS/MS analysis | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50359988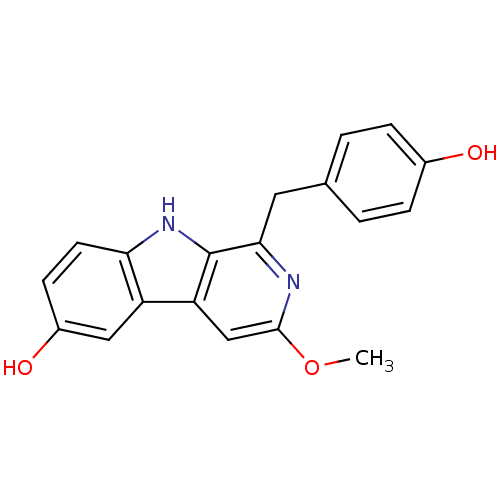 (CHEMBL1927942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reagent method | J Nat Prod 74: 2489-96 (2011) Article DOI: 10.1021/np100874f BindingDB Entry DOI: 10.7270/Q2ST7Q8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479075 (CHEMBL492770) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase 3'-processing activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electrophore... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50312648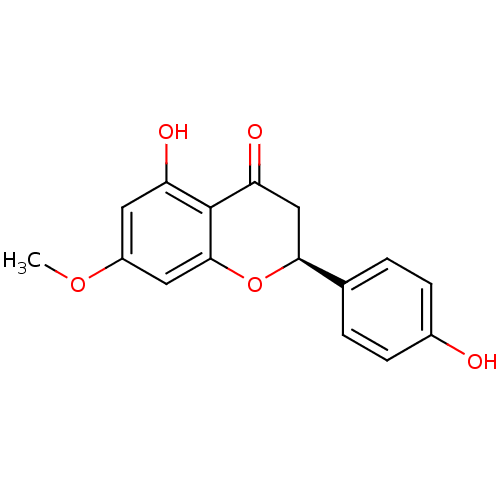 ((2S)-5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-2,3-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reagent method | J Nat Prod 74: 2489-96 (2011) Article DOI: 10.1021/np100874f BindingDB Entry DOI: 10.7270/Q2ST7Q8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479066 (CHEMBL454190) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase 3'-processing activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electrophore... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50509618 (CHEMBL4463663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 using S-mephenytoin as substrate by LC/MS/MS analysis | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479049 (CHEMBL467389) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479053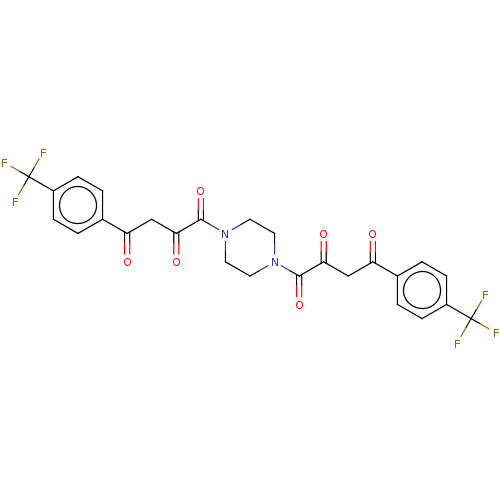 (CHEMBL464340) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479051 (CHEMBL468234) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479058 (CHEMBL467380) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase 3'-processing activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electrophore... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479052 (CHEMBL468233) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50207159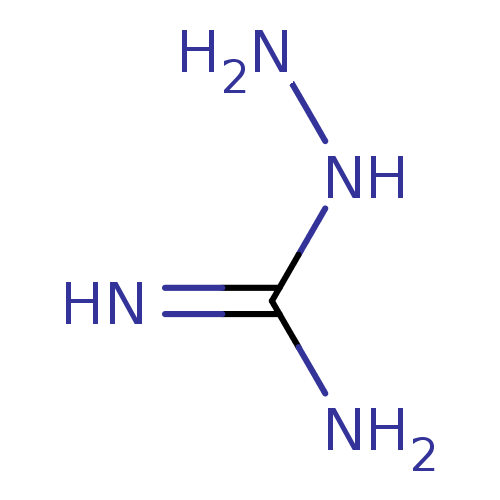 (2-aminoguanidine | 2-azanylguanidine | AMINOGUANID...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS in mouse RAW264.7 cells assessed as anti-inflammatory activity by measuring maximum inhibition of LPS-induced nitric oxide product... | J Nat Prod 74: 1875-80 (2011) Article DOI: 10.1021/np200279r BindingDB Entry DOI: 10.7270/Q2474B72 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479079 (CHEMBL502238) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479108 (CHEMBL512205) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type integrase strand transfer activity | Bioorg Med Chem Lett 18: 4521-4 (2008) Article DOI: 10.1016/j.bmcl.2008.07.047 BindingDB Entry DOI: 10.7270/Q2BV7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50509618 (CHEMBL4463663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 using sorafenib as substrate by LC/MS/MS analysis | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50207159 (2-aminoguanidine | 2-azanylguanidine | AMINOGUANID...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibition of iNOS-mediated NO production in LPS-stimulated mouse RAW264.7 cells after 24 hrs by Griess reagent method | J Nat Prod 74: 2489-96 (2011) Article DOI: 10.1021/np100874f BindingDB Entry DOI: 10.7270/Q2ST7Q8Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479080 (CHEMBL467360) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479071 (CHEMBL513666) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479055 (CHEMBL469071) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50509618 (CHEMBL4463663) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 using dextromethorphan as substrate by LC/MS/MS analysis | ACS Med Chem Lett 11: 166-171 (2020) Article DOI: 10.1021/acsmedchemlett.9b00550 BindingDB Entry DOI: 10.7270/Q2RF5Z9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50479070 (CHEMBL465864) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 integrase strand transfer activity using [32P]-labeled linear oligonucleotide substrate by polyacrylamide gel electropho... | Bioorg Med Chem 16: 7777-87 (2008) Article DOI: 10.1016/j.bmc.2008.07.008 BindingDB Entry DOI: 10.7270/Q2M90CG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 121 total ) | Next | Last >> |