 Found 222 hits with Last Name = 'guillaume' and Initial = 'j'
Found 222 hits with Last Name = 'guillaume' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50017721

(1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2ccccc2-[#6]=[#6]-c2ccccc-12 |c:16| Show InChI InChI=1S/C21H21N/c1-22-14-12-18(13-15-22)21-19-8-4-2-6-16(19)10-11-17-7-3-5-9-20(17)21/h2-11H,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50017721

(1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2ccccc2-[#6]=[#6]-c2ccccc-12 |c:16| Show InChI InChI=1S/C21H21N/c1-22-14-12-18(13-15-22)21-19-8-4-2-6-16(19)10-11-17-7-3-5-9-20(17)21/h2-11H,12-15H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50292411
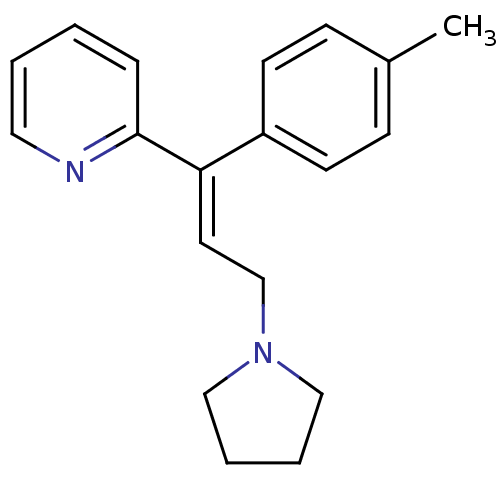
((E)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)py...)Show InChI InChI=1S/C19H22N2/c1-16-7-9-17(10-8-16)18(19-6-2-3-12-20-19)11-15-21-13-4-5-14-21/h2-3,6-12H,4-5,13-15H2,1H3/b18-11+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM35938
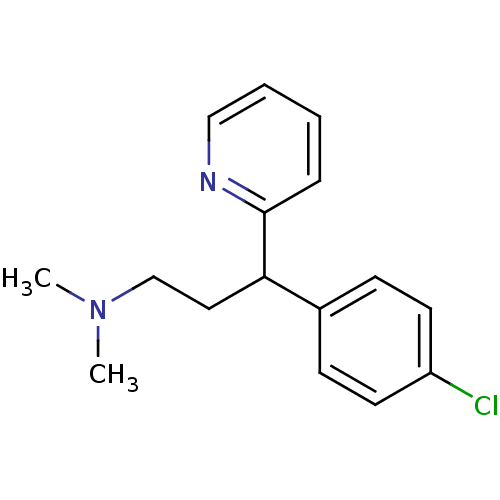
(1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...)Show InChI InChI=1S/C16H19ClN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50292411

((E)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)py...)Show InChI InChI=1S/C19H22N2/c1-16-7-9-17(10-8-16)18(19-6-2-3-12-20-19)11-15-21-13-4-5-14-21/h2-3,6-12H,4-5,13-15H2,1H3/b18-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50292411

((E)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)py...)Show InChI InChI=1S/C19H22N2/c1-16-7-9-17(10-8-16)18(19-6-2-3-12-20-19)11-15-21-13-4-5-14-21/h2-3,6-12H,4-5,13-15H2,1H3/b18-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM35938

(1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...)Show InChI InChI=1S/C16H19ClN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM35938

(1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...)Show InChI InChI=1S/C16H19ClN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22879
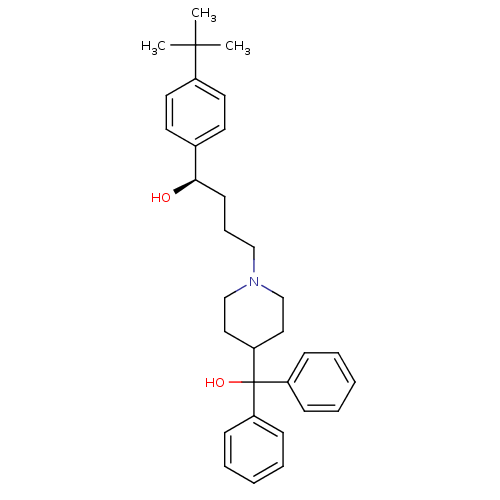
((1R)-1-(4-tert-butylphenyl)-4-[4-(hydroxydiphenylm...)Show SMILES CC(C)(C)c1ccc(cc1)[C@H](O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM85029
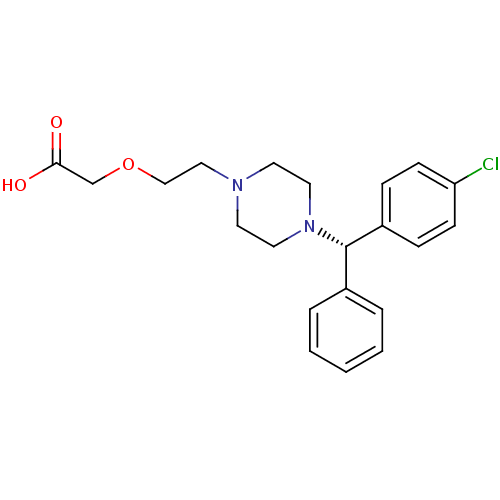
(Cetirizine (+) isomer)Show SMILES OC(=O)COCCN1CCN(CC1)[C@@H](c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM22890

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show SMILES OC(=O)COCCN1CCN(CC1)C(c1ccccc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM85030

(Cetirizine (-) isomer)Show SMILES OC(=O)COCCN1CCN(CC1)[C@H](c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394918
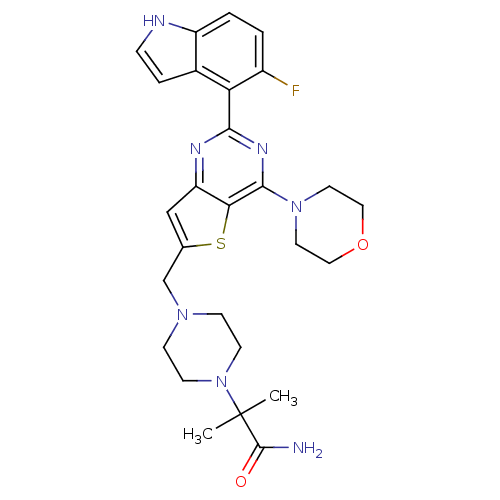
(CHEMBL2165504)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-9-7-33(8-10-35)16-17-15-21-23(38-17)25(34-11-13-37-14-12-34)32-24(31-21)22-18-5-6-30-20(18)4-3-19(22)28/h3-6,15,30H,7-14,16H2,1-2H3,(H2,29,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22893
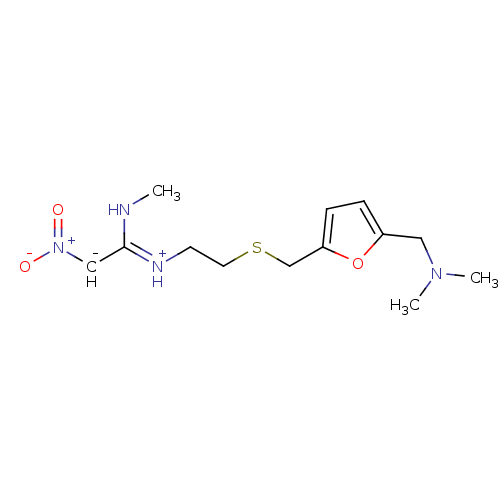
(CHEMBL512 | Ranitidine | ZANTAC | dimethyl[(5-{[(2...)Show SMILES CN\C([CH-][N+]([O-])=O)=[NH+]/CCSCc1ccc(CN(C)C)o1 Show InChI InChI=1S/C13H21N4O3S/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3/h4-5,9H,6-8,10H2,1-3H3,(H,14,15)/q-1/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles
Curated by PDSP Ki Database
| |
Eur J Biochem 224: 489-95 (1994)
Article DOI: 10.1111/j.1432-1033.1994.00489.x
BindingDB Entry DOI: 10.7270/Q2H70DB4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394917
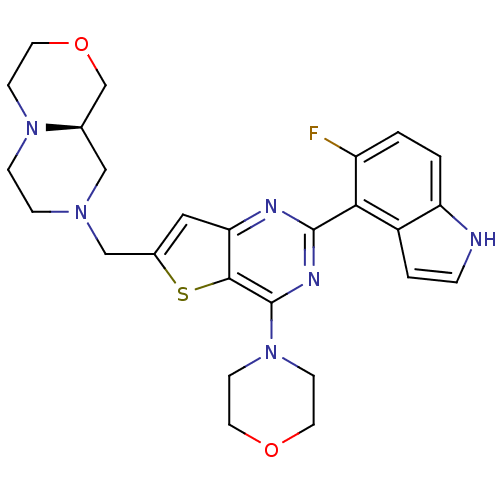
(CHEMBL2165505)Show SMILES Fc1ccc2[nH]ccc2c1-c1nc(N2CCOCC2)c2sc(CN3CCN4CCOC[C@H]4C3)cc2n1 |r| Show InChI InChI=1S/C26H29FN6O2S/c27-20-1-2-21-19(3-4-28-21)23(20)25-29-22-13-18(15-31-5-6-32-7-12-35-16-17(32)14-31)36-24(22)26(30-25)33-8-10-34-11-9-33/h1-4,13,17,28H,5-12,14-16H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470233
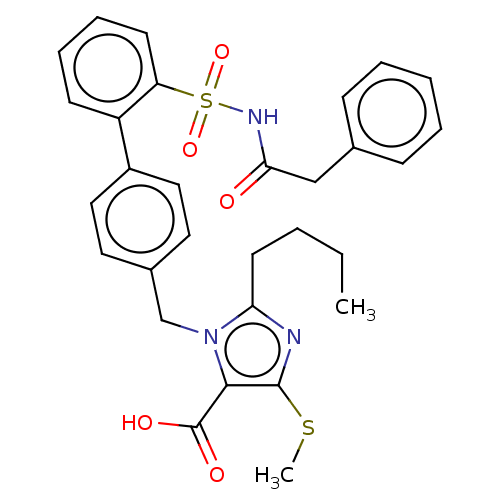
(CHEMBL80177)Show SMILES CCCCc1nc(SC)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)Cc1ccccc1 Show InChI InChI=1S/C30H31N3O5S2/c1-3-4-14-26-31-29(39-2)28(30(35)36)33(26)20-22-15-17-23(18-16-22)24-12-8-9-13-25(24)40(37,38)32-27(34)19-21-10-6-5-7-11-21/h5-13,15-18H,3-4,14,19-20H2,1-2H3,(H,32,34)(H,35,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470229
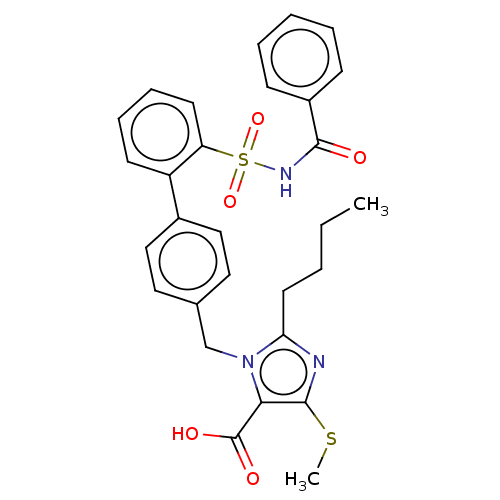
(CHEMBL76870)Show SMILES CCCCc1nc(SC)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C29H29N3O5S2/c1-3-4-14-25-30-28(38-2)26(29(34)35)32(25)19-20-15-17-21(18-16-20)23-12-8-9-13-24(23)39(36,37)31-27(33)22-10-6-5-7-11-22/h5-13,15-18H,3-4,14,19H2,1-2H3,(H,31,33)(H,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470236
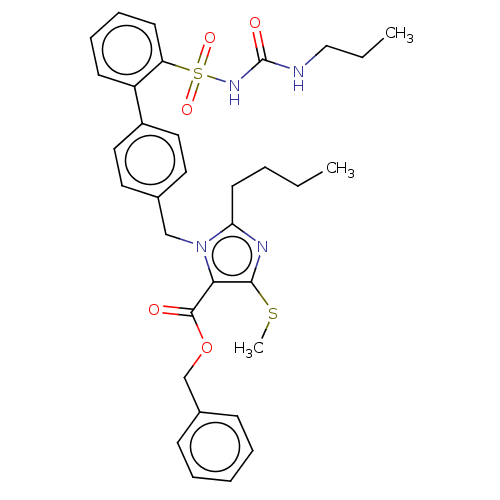
(CHEMBL309313)Show SMILES CCCCc1nc(SC)c(C(=O)OCc2ccccc2)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C33H38N4O5S2/c1-4-6-16-29-35-31(43-3)30(32(38)42-23-25-12-8-7-9-13-25)37(29)22-24-17-19-26(20-18-24)27-14-10-11-15-28(27)44(40,41)36-33(39)34-21-5-2/h7-15,17-20H,4-6,16,21-23H2,1-3H3,(H2,34,36,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470239
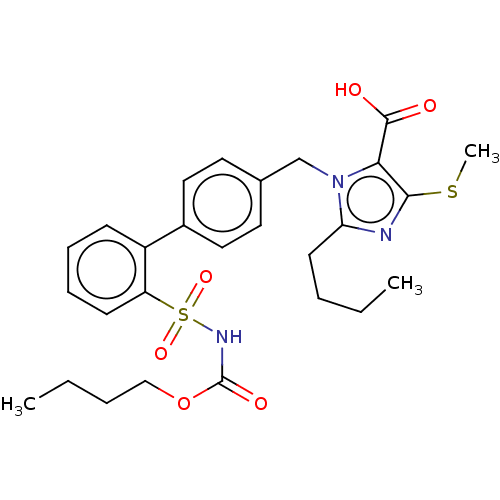
(CHEMBL311312)Show SMILES CCCCOC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCCC)nc(SC)c2C(O)=O)cc1 Show InChI InChI=1S/C27H33N3O6S2/c1-4-6-12-23-28-25(37-3)24(26(31)32)30(23)18-19-13-15-20(16-14-19)21-10-8-9-11-22(21)38(34,35)29-27(33)36-17-7-5-2/h8-11,13-16H,4-7,12,17-18H2,1-3H3,(H,29,33)(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285769

(5-alpha-hydroxyacid imidazolyl biphenyl sulfonyl u...)Show SMILES CCCCc1nc(SC)c(C(=O)n2cnnn2)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCc1ccccc1 Show InChI InChI=1S/C31H32N8O4S2/c1-3-4-14-27-34-29(44-2)28(30(40)39-21-33-36-37-39)38(27)20-23-15-17-24(18-16-23)25-12-8-9-13-26(25)45(42,43)35-31(41)32-19-22-10-6-5-7-11-22/h5-13,15-18,21H,3-4,14,19-20H2,1-2H3,(H2,32,35,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II Type 1 receptor in rat liver membrane by [125I]AII displacement. |
Bioorg Med Chem Lett 5: 2611-2616 (1995)
Article DOI: 10.1016/0960-894X(95)00479-D
BindingDB Entry DOI: 10.7270/Q2GT5N57 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285761

(5-alpha-hydroxyacid imidazolyl biphenyl sulfonyl u...)Show SMILES CCCCc1nc(SC)c(C(=O)C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCc1ccccc1 Show InChI InChI=1S/C31H32N4O6S2/c1-3-4-14-26-33-29(42-2)27(28(36)30(37)38)35(26)20-22-15-17-23(18-16-22)24-12-8-9-13-25(24)43(40,41)34-31(39)32-19-21-10-6-5-7-11-21/h5-13,15-18H,3-4,14,19-20H2,1-2H3,(H,37,38)(H2,32,34,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II Type 1 receptor in rat liver membrane by [125I]AII displacement. |
Bioorg Med Chem Lett 5: 2611-2616 (1995)
Article DOI: 10.1016/0960-894X(95)00479-D
BindingDB Entry DOI: 10.7270/Q2GT5N57 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285760
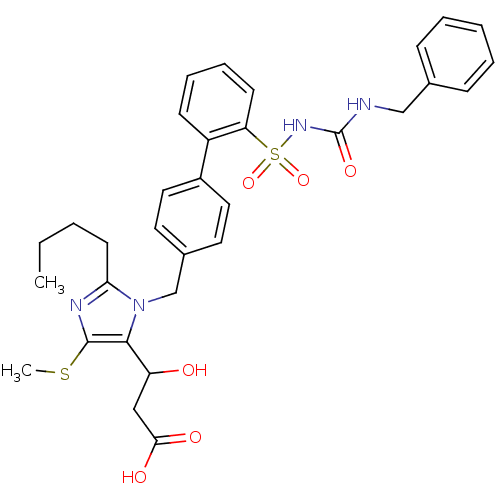
(5-alpha-hydroxyacid imidazolyl biphenyl sulfonyl u...)Show SMILES CCCCc1nc(SC)c(C(O)CC(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCc1ccccc1 Show InChI InChI=1S/C32H36N4O6S2/c1-3-4-14-28-34-31(43-2)30(26(37)19-29(38)39)36(28)21-23-15-17-24(18-16-23)25-12-8-9-13-27(25)44(41,42)35-32(40)33-20-22-10-6-5-7-11-22/h5-13,15-18,26,37H,3-4,14,19-21H2,1-2H3,(H,38,39)(H2,33,35,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II Type 1 receptor in rat liver membrane by [125I]AII displacement. |
Bioorg Med Chem Lett 5: 2611-2616 (1995)
Article DOI: 10.1016/0960-894X(95)00479-D
BindingDB Entry DOI: 10.7270/Q2GT5N57 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50031491

(2-propyl-4-(methythio)-1-[[[2'-[(propylamino)carbo...)Show SMILES CCCNC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(SC)c2C(O)=O)cc1 Show InChI InChI=1S/C25H30N4O5S2/c1-4-8-21-27-23(35-3)22(24(30)31)29(21)16-17-11-13-18(14-12-17)19-9-6-7-10-20(19)36(33,34)28-25(32)26-15-5-2/h6-7,9-14H,4-5,8,15-16H2,1-3H3,(H,30,31)(H2,26,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50213557
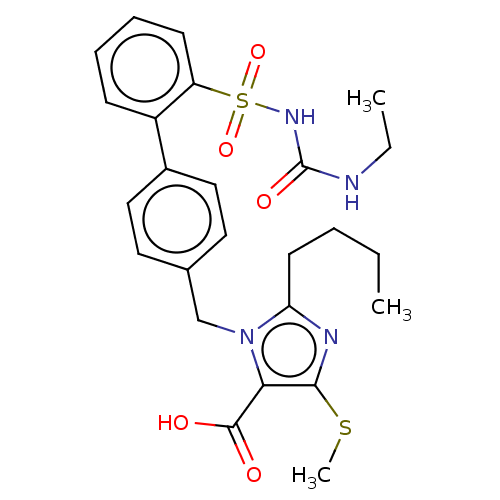
(CHEMBL309602)Show SMILES CCCCc1nc(SC)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCC Show InChI InChI=1S/C25H30N4O5S2/c1-4-6-11-21-27-23(35-3)22(24(30)31)29(21)16-17-12-14-18(15-13-17)19-9-7-8-10-20(19)36(33,34)28-25(32)26-5-2/h7-10,12-15H,4-6,11,16H2,1-3H3,(H,30,31)(H2,26,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470250
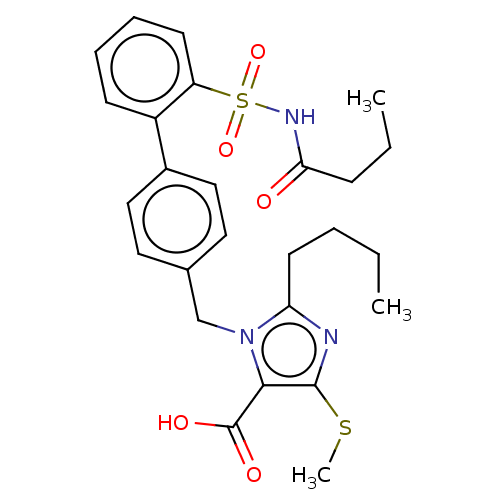
(CHEMBL77308)Show SMILES CCCCc1nc(SC)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)CCC Show InChI InChI=1S/C26H31N3O5S2/c1-4-6-12-22-27-25(35-3)24(26(31)32)29(22)17-18-13-15-19(16-14-18)20-10-7-8-11-21(20)36(33,34)28-23(30)9-5-2/h7-8,10-11,13-16H,4-6,9,12,17H2,1-3H3,(H,28,30)(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470206
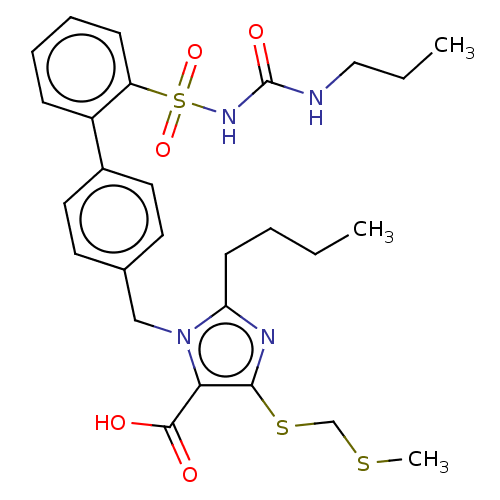
(CHEMBL445365)Show SMILES CCCCc1nc(SCSC)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C27H34N4O5S3/c1-4-6-11-23-29-25(38-18-37-3)24(26(32)33)31(23)17-19-12-14-20(15-13-19)21-9-7-8-10-22(21)39(35,36)30-27(34)28-16-5-2/h7-10,12-15H,4-6,11,16-18H2,1-3H3,(H,32,33)(H2,28,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50213569

(CHEMBL78866)Show SMILES CCCCNC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCCC)nc(SC)c2C(O)=O)cc1 Show InChI InChI=1S/C27H34N4O5S2/c1-4-6-12-23-29-25(37-3)24(26(32)33)31(23)18-19-13-15-20(16-14-19)21-10-8-9-11-22(21)38(35,36)30-27(34)28-17-7-5-2/h8-11,13-16H,4-7,12,17-18H2,1-3H3,(H,32,33)(H2,28,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470225
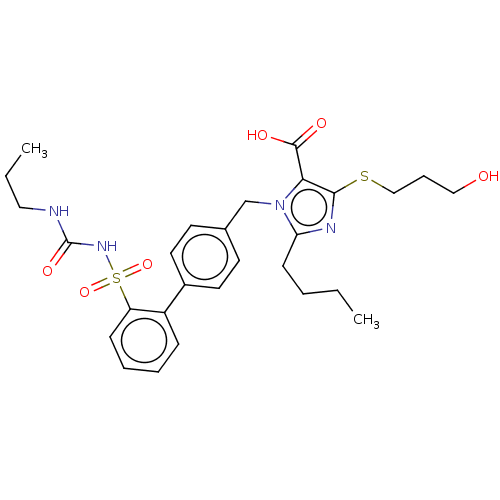
(CHEMBL80700)Show SMILES CCCCc1nc(SCCCO)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C28H36N4O6S2/c1-3-5-11-24-30-26(39-18-8-17-33)25(27(34)35)32(24)19-20-12-14-21(15-13-20)22-9-6-7-10-23(22)40(37,38)31-28(36)29-16-4-2/h6-7,9-10,12-15,33H,3-5,8,11,16-19H2,1-2H3,(H,34,35)(H2,29,31,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470226
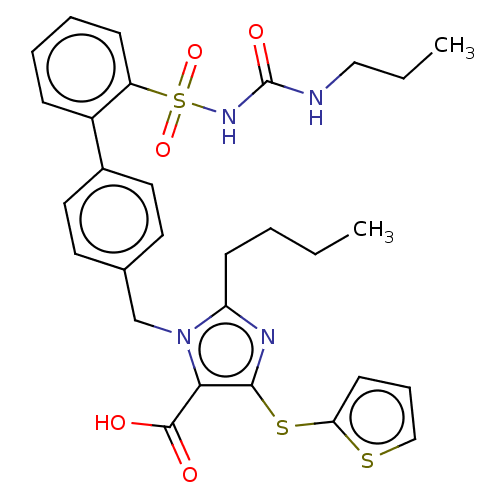
(CHEMBL311827)Show SMILES CCCCc1nc(Sc2cccs2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C29H32N4O5S3/c1-3-5-11-24-31-27(40-25-12-8-18-39-25)26(28(34)35)33(24)19-20-13-15-21(16-14-20)22-9-6-7-10-23(22)41(37,38)32-29(36)30-17-4-2/h6-10,12-16,18H,3-5,11,17,19H2,1-2H3,(H,34,35)(H2,30,32,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470235

(CHEMBL308135)Show SMILES CCCCc1nc(SC)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)OCC(C)C Show InChI InChI=1S/C27H33N3O6S2/c1-5-6-11-23-28-25(37-4)24(26(31)32)30(23)16-19-12-14-20(15-13-19)21-9-7-8-10-22(21)38(34,35)29-27(33)36-17-18(2)3/h7-10,12-15,18H,5-6,11,16-17H2,1-4H3,(H,29,33)(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285767
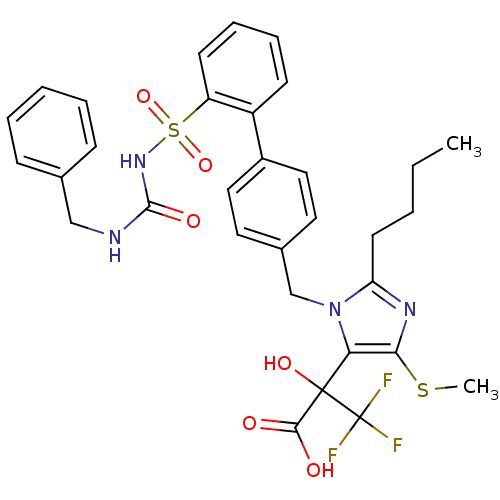
(5-alpha-hydroxyacid imidazolyl biphenyl sulfonyl u...)Show SMILES CCCCc1nc(SC)c(n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCc1ccccc1)C(O)(C(O)=O)C(F)(F)F Show InChI InChI=1S/C32H33F3N4O6S2/c1-3-4-14-26-37-28(46-2)27(31(43,29(40)41)32(33,34)35)39(26)20-22-15-17-23(18-16-22)24-12-8-9-13-25(24)47(44,45)38-30(42)36-19-21-10-6-5-7-11-21/h5-13,15-18,43H,3-4,14,19-20H2,1-2H3,(H,40,41)(H2,36,38,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II Type 1 receptor in rat liver membrane by [125I]AII displacement. |
Bioorg Med Chem Lett 5: 2611-2616 (1995)
Article DOI: 10.1016/0960-894X(95)00479-D
BindingDB Entry DOI: 10.7270/Q2GT5N57 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470241

(CHEMBL305638)Show SMILES CCCCc1nc(SCCCCOC(C)=O)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C31H40N4O7S2/c1-4-6-13-27-33-29(43-20-10-9-19-42-22(3)36)28(30(37)38)35(27)21-23-14-16-24(17-15-23)25-11-7-8-12-26(25)44(40,41)34-31(39)32-18-5-2/h7-8,11-12,14-17H,4-6,9-10,13,18-21H2,1-3H3,(H,37,38)(H2,32,34,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470227
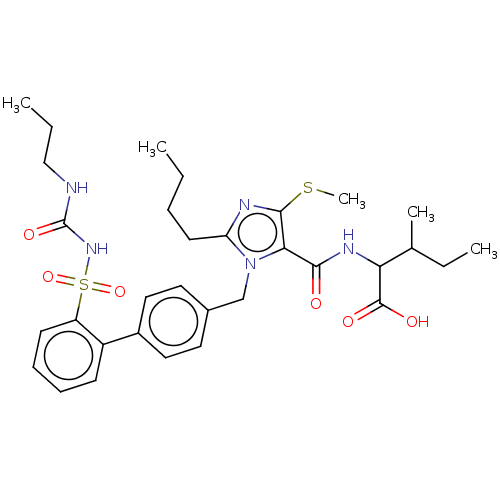
(CHEMBL76538)Show SMILES CCCCc1nc(SC)c(C(=O)NC(C(C)CC)C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C32H43N5O6S2/c1-6-9-14-26-34-30(44-5)28(29(38)35-27(31(39)40)21(4)8-3)37(26)20-22-15-17-23(18-16-22)24-12-10-11-13-25(24)45(42,43)36-32(41)33-19-7-2/h10-13,15-18,21,27H,6-9,14,19-20H2,1-5H3,(H,35,38)(H,39,40)(H2,33,36,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470249

(CHEMBL309845)Show SMILES CCCCc1nc(SCC(F)(F)C(F)(F)CO)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C29H34F4N4O6S2/c1-3-5-10-23-35-25(44-18-29(32,33)28(30,31)17-38)24(26(39)40)37(23)16-19-11-13-20(14-12-19)21-8-6-7-9-22(21)45(42,43)36-27(41)34-15-4-2/h6-9,11-14,38H,3-5,10,15-18H2,1-2H3,(H,39,40)(H2,34,36,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50031537

(2-Butyl-3-(N'-propylureidylsulfonyl-biphenyl-4-ylm...)Show SMILES CCCCc1nc(SC)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C26H32N4O5S2/c1-4-6-11-22-28-24(36-3)23(25(31)32)30(22)17-18-12-14-19(15-13-18)20-9-7-8-10-21(20)37(34,35)29-26(33)27-16-5-2/h7-10,12-15H,4-6,11,16-17H2,1-3H3,(H,31,32)(H2,27,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470223
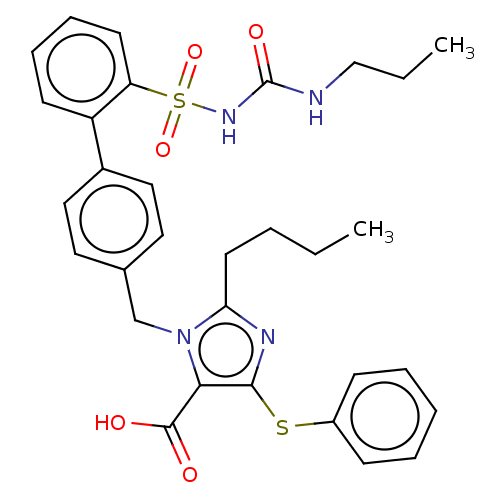
(CHEMBL312386)Show SMILES CCCCc1nc(Sc2ccccc2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C31H34N4O5S2/c1-3-5-15-27-33-29(41-24-11-7-6-8-12-24)28(30(36)37)35(27)21-22-16-18-23(19-17-22)25-13-9-10-14-26(25)42(39,40)34-31(38)32-20-4-2/h6-14,16-19H,3-5,15,20-21H2,1-2H3,(H,36,37)(H2,32,34,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470228
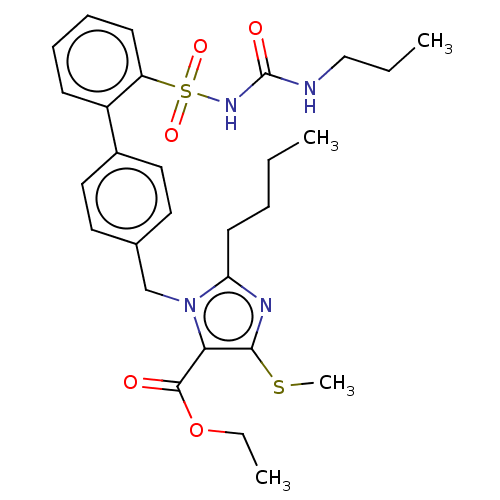
(CHEMBL80971)Show SMILES CCCCc1nc(SC)c(C(=O)OCC)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C28H36N4O5S2/c1-5-8-13-24-30-26(38-4)25(27(33)37-7-3)32(24)19-20-14-16-21(17-15-20)22-11-9-10-12-23(22)39(35,36)31-28(34)29-18-6-2/h9-12,14-17H,5-8,13,18-19H2,1-4H3,(H2,29,31,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50285752
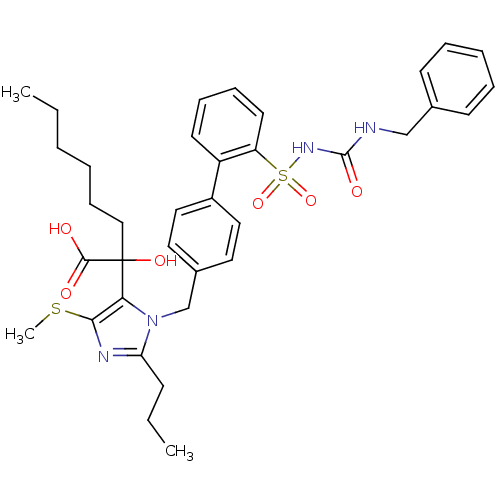
(5-alpha-hydroxyacid imidazolyl biphenyl sulfonyl u...)Show SMILES CCCCCCC(O)(C(O)=O)c1c(SC)nc(CCC)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCc1ccccc1 Show InChI InChI=1S/C36H44N4O6S2/c1-4-6-7-13-23-36(44,34(41)42)32-33(47-3)38-31(14-5-2)40(32)25-27-19-21-28(22-20-27)29-17-11-12-18-30(29)48(45,46)39-35(43)37-24-26-15-9-8-10-16-26/h8-12,15-22,44H,4-7,13-14,23-25H2,1-3H3,(H,41,42)(H2,37,39,43) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II Type 1 receptor in rat liver membrane by [125I]AII displacement. |
Bioorg Med Chem Lett 5: 2611-2616 (1995)
Article DOI: 10.1016/0960-894X(95)00479-D
BindingDB Entry DOI: 10.7270/Q2GT5N57 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470234
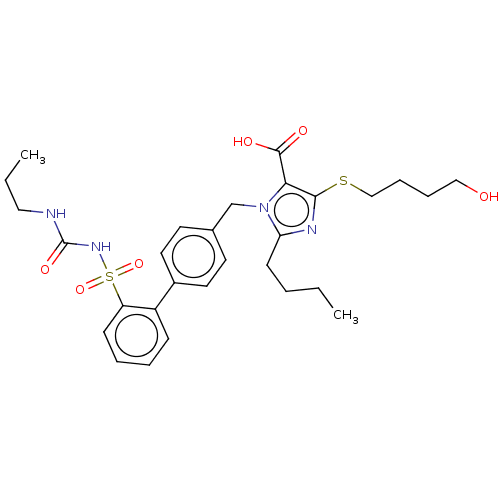
(CHEMBL76948)Show SMILES CCCCc1nc(SCCCCO)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C29H38N4O6S2/c1-3-5-12-25-31-27(40-19-9-8-18-34)26(28(35)36)33(25)20-21-13-15-22(16-14-21)23-10-6-7-11-24(23)41(38,39)32-29(37)30-17-4-2/h6-7,10-11,13-16,34H,3-5,8-9,12,17-20H2,1-2H3,(H,35,36)(H2,30,32,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50031530
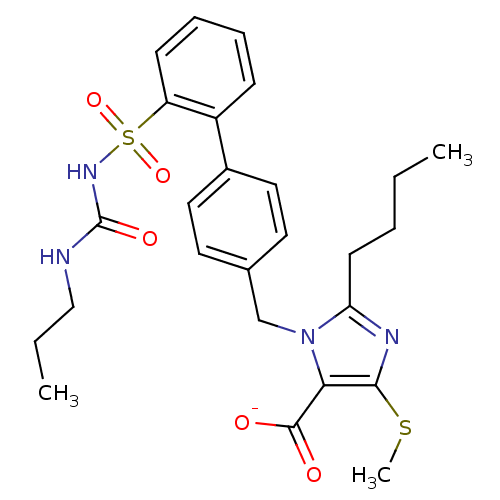
(2-Butyl-4-(methylthio)-1-[[2'-[[[(propylamino)carb...)Show SMILES CCCCc1nc(SC)c(C([O-])=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C26H32N4O5S2/c1-4-6-11-22-28-24(36-3)23(25(31)32)30(22)17-18-12-14-19(15-13-18)20-9-7-8-10-21(20)37(34,35)29-26(33)27-16-5-2/h7-10,12-15H,4-6,11,16-17H2,1-3H3,(H,31,32)(H2,27,29,33)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394916
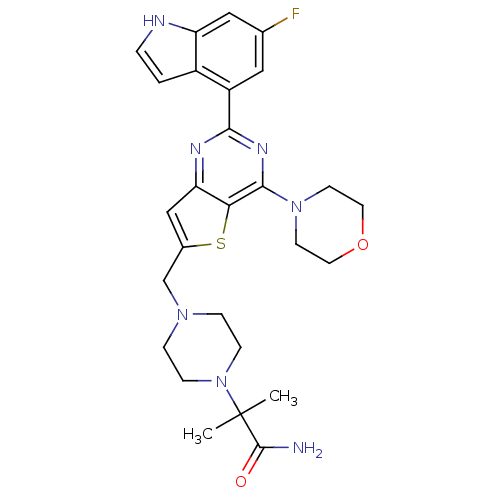
(CHEMBL2165506)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cc(F)cc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-7-5-33(6-8-35)16-18-15-22-23(38-18)25(34-9-11-37-12-10-34)32-24(31-22)20-13-17(28)14-21-19(20)3-4-30-21/h3-4,13-15,30H,5-12,16H2,1-2H3,(H2,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50470243

(CHEMBL306157)Show SMILES CCCCc1nc(SC(F)F)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C26H30F2N4O5S2/c1-3-5-10-21-30-23(38-25(27)28)22(24(33)34)32(21)16-17-11-13-18(14-12-17)19-8-6-7-9-20(19)39(36,37)31-26(35)29-15-4-2/h6-9,11-14,25H,3-5,10,15-16H2,1-2H3,(H,33,34)(H2,29,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents
Curated by ChEMBL
| Assay Description
Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane |
J Med Chem 38: 2357-77 (1995)
Article DOI: 10.1021/jm00013a013
BindingDB Entry DOI: 10.7270/Q2KK9FH8 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50031530

(2-Butyl-4-(methylthio)-1-[[2'-[[[(propylamino)carb...)Show SMILES CCCCc1nc(SC)c(C([O-])=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)NCCC Show InChI InChI=1S/C26H32N4O5S2/c1-4-6-11-22-28-24(36-3)23(25(31)32)30(22)17-18-12-14-19(15-13-18)20-9-7-8-10-21(20)37(34,35)29-26(33)27-16-5-2/h7-10,12-15H,4-6,11,16-17H2,1-3H3,(H,31,32)(H2,27,29,33)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II Type 1 receptor in rat liver membrane by [125I]AII displacement. |
Bioorg Med Chem Lett 5: 2611-2616 (1995)
Article DOI: 10.1016/0960-894X(95)00479-D
BindingDB Entry DOI: 10.7270/Q2GT5N57 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data