

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50480998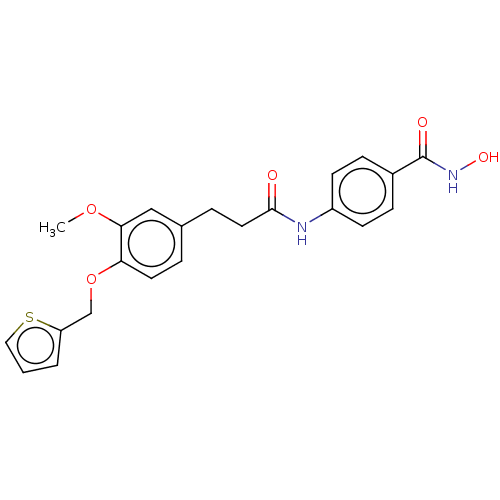 (CHEMBL567972) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481005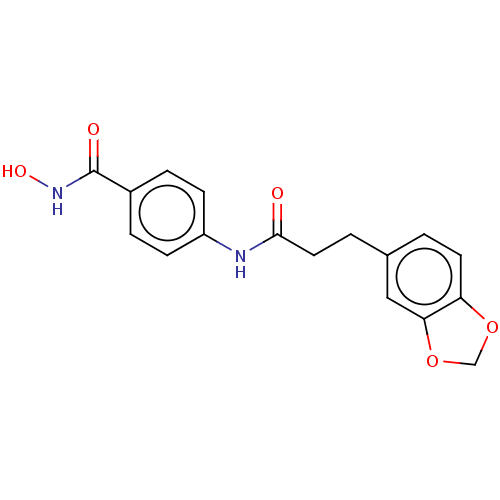 (CHEMBL568586) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308316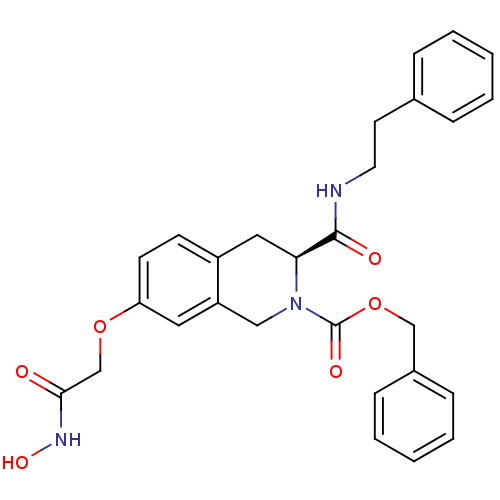 ((S)-Benzyl 7-(2-(hydroxyamino)-2-oxoethoxy)-3-(phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50480995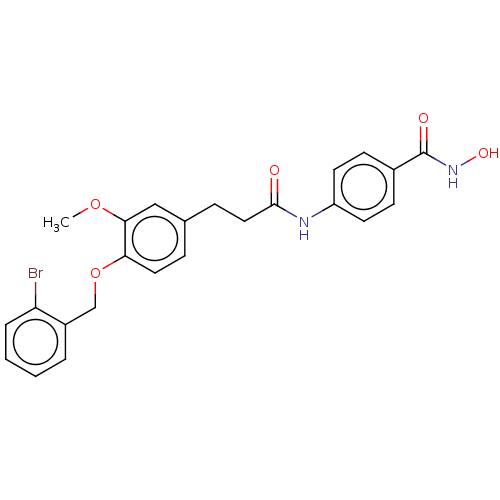 (CHEMBL570280) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481012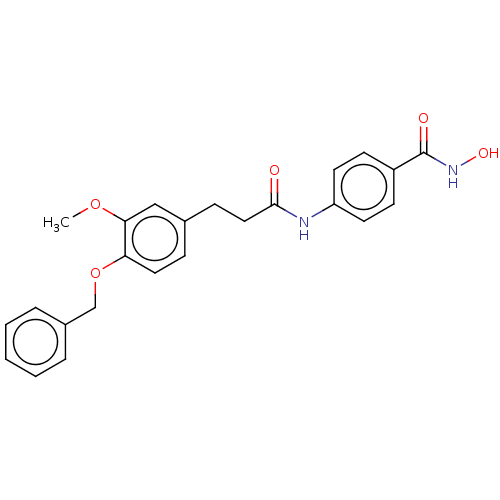 (CHEMBL570499) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481007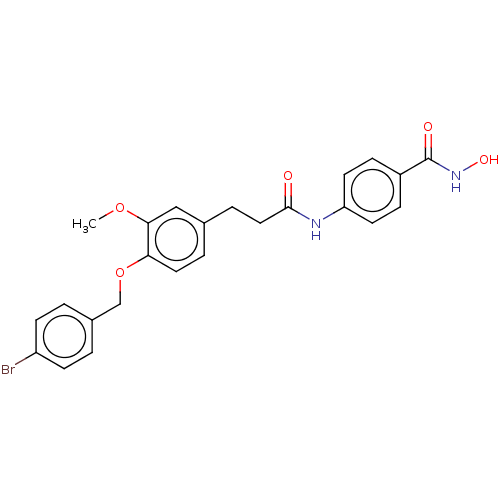 (CHEMBL568184) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481011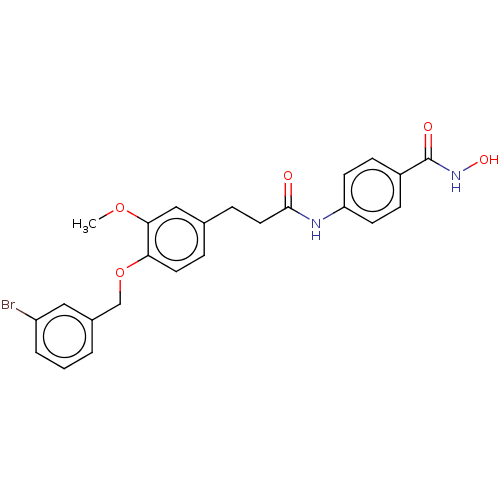 (CHEMBL571358) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308290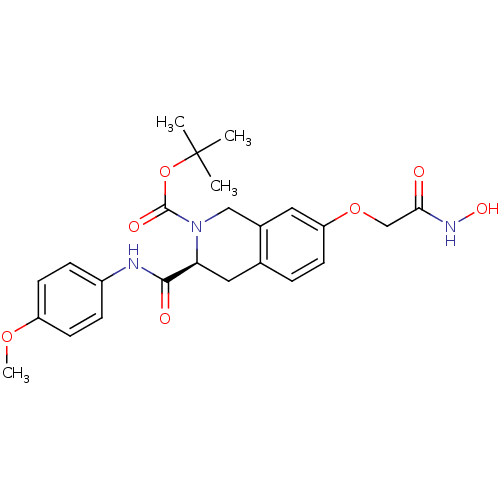 ((S)-tert-Butyl 7-(2-(hydroxyamino)-2-oxoethoxy)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308307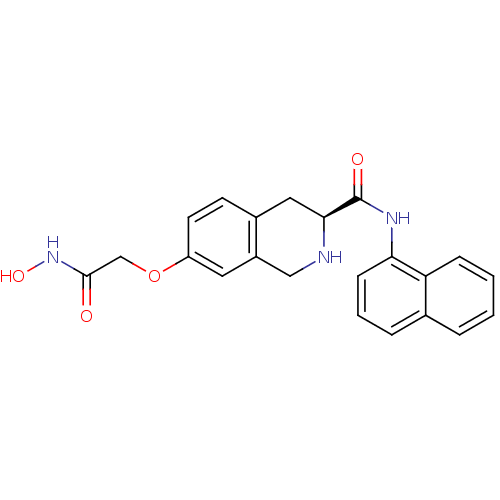 ((S)-7-(2-(Hydroxyamino)-2-oxoethoxy)-N-(naphthalen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shan Dong University | Assay Description In vitro bioactivity evaluation of compounds 4a-n was performed by HDAC activity assays using a HDAC colorimetric activity assay kit (AK501; Biomol R... | J Enzyme Inhib Med Chem 25: 132-8 (2010) Article DOI: 10.3109/14756360903049034 BindingDB Entry DOI: 10.7270/Q2MS3RNW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50475234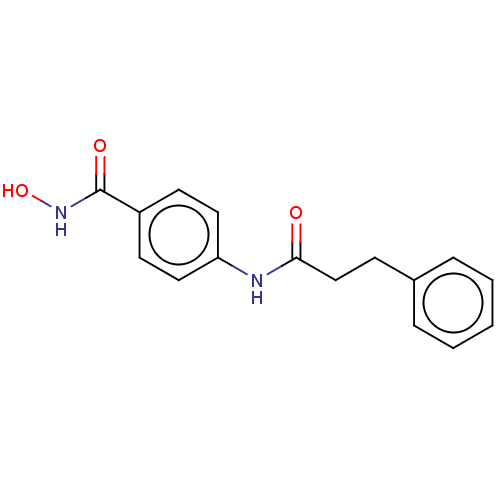 (CHEMBL189912) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308300 ((S)-tert-Butyl 3-(3-chlorophenylcarbamoyl)-7-(2-(h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50480996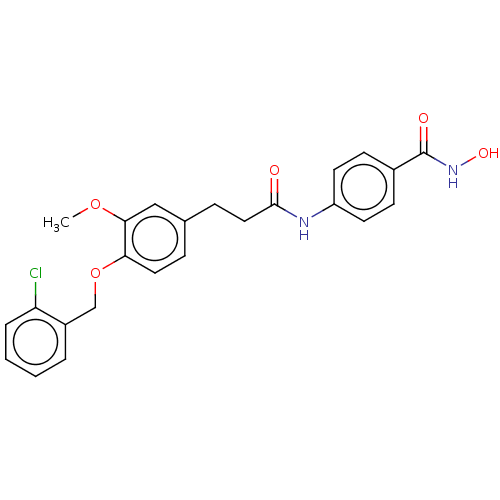 (CHEMBL568185) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308284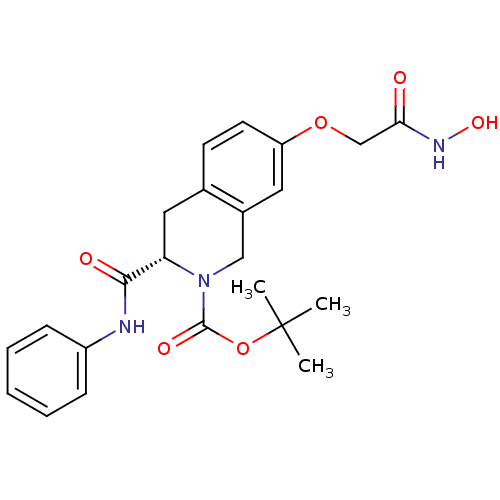 ((S)-tert-Butyl 7-(2-(hydroxyamino)-2-oxoethoxy)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50480994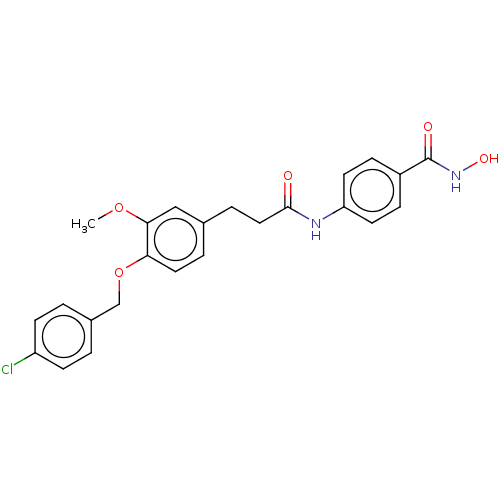 (CHEMBL586060) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481010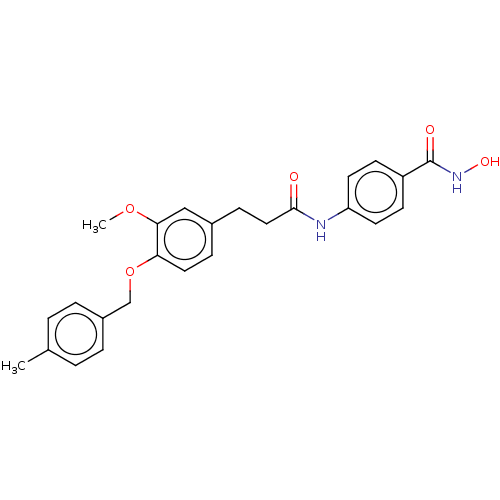 (CHEMBL570279) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308304 ((S)-tert-Butyl 3-(3-chloro-4-fluorophenylcarbamoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481001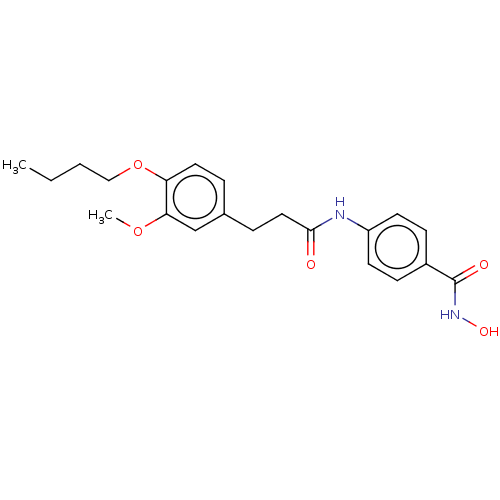 (CHEMBL578209) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50480993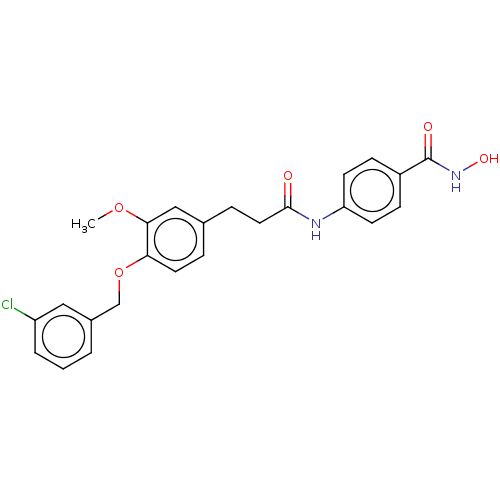 (CHEMBL567155) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308292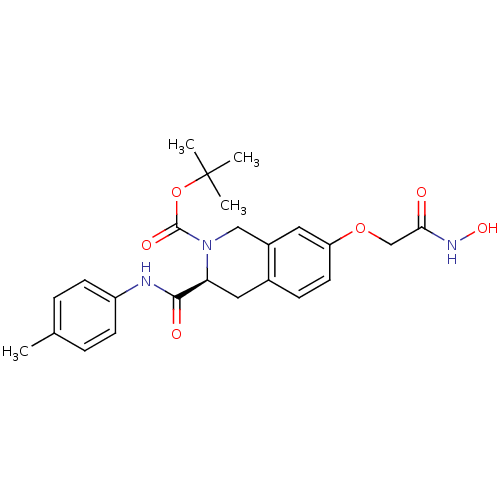 ((S)-tert-Butyl 7-(2-(hydroxyamino)-2-oxoethoxy)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481009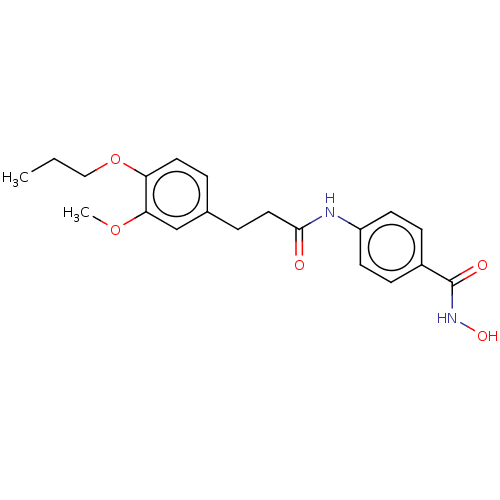 (CHEMBL571802) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308296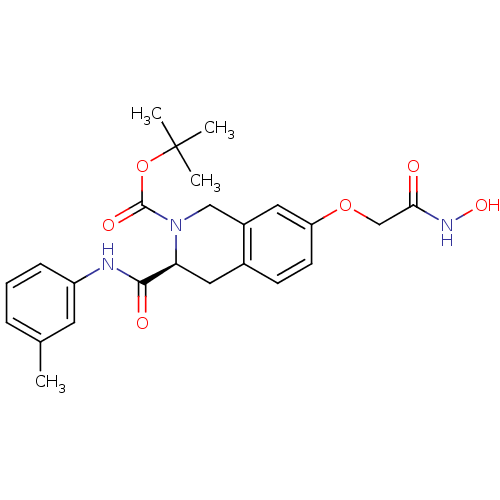 ((S)-tert-Butyl 7-(2-(hydroxyamino)-2-oxoethoxy)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481002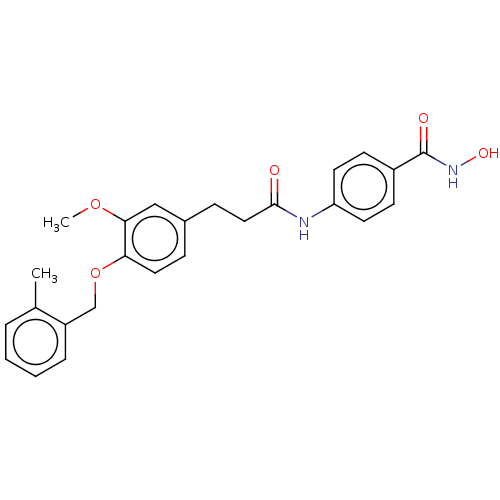 (CHEMBL585905) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308308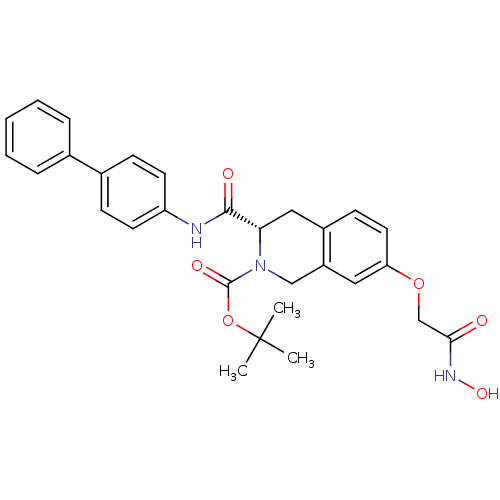 ((S)-tert-Butyl 3-(biphenyl-4-ylcarbamoyl)-7-(2-(hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481004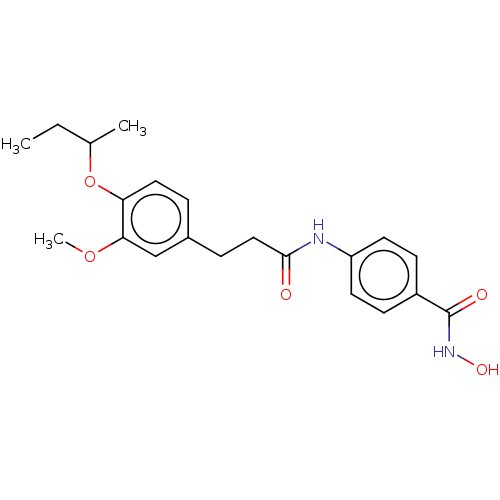 (CHEMBL576109) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308309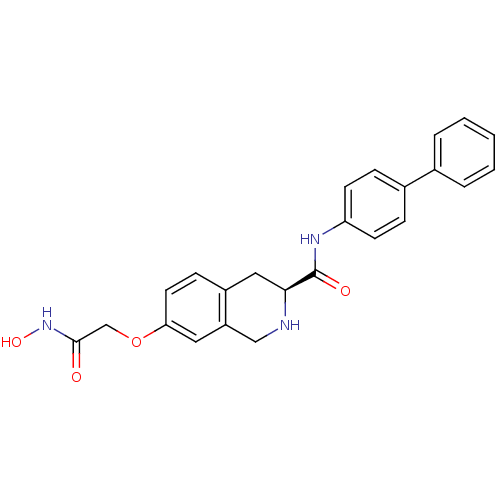 ((S)-N-(Biphenyl-4-yl)-7-(2-(hydroxyamino)-2-oxoeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308298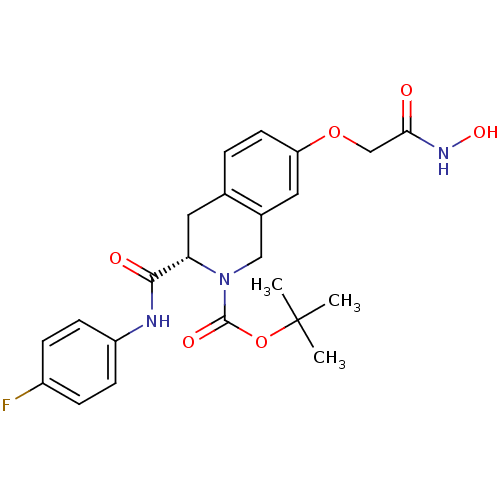 ((S)-tert-Butyl 3-(4-fluorophenylcarbamoyl)-7-(2-(h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481006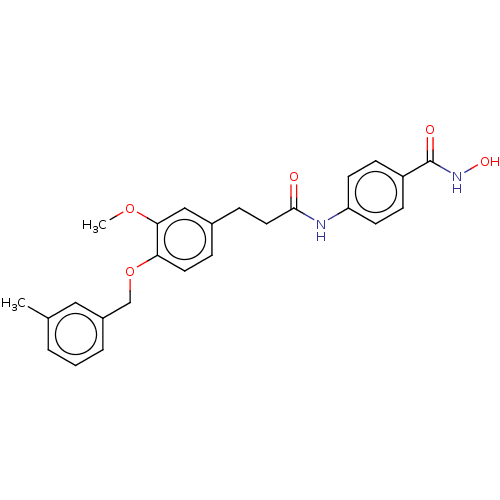 (CHEMBL570492) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308288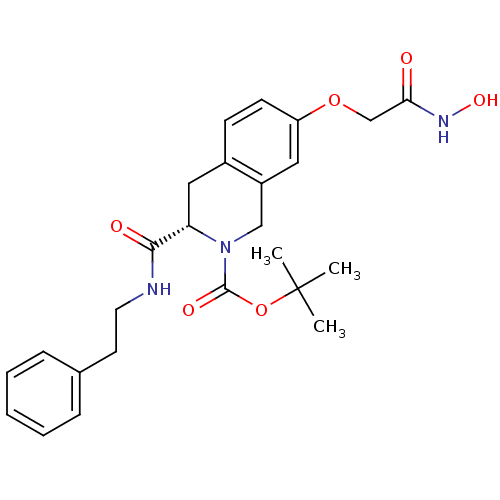 ((S)-tert-Butyl 7-(2-(hydroxyamino)-2-oxoethoxy)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50480997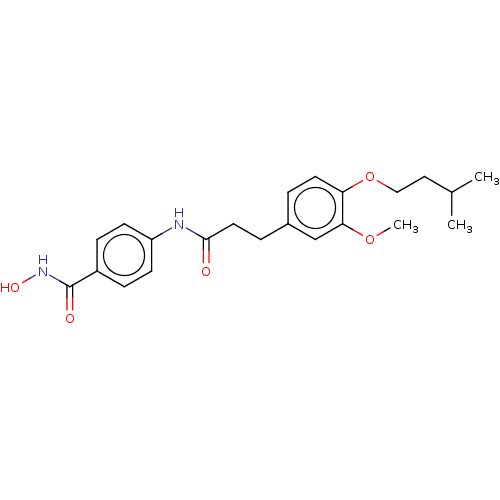 (CHEMBL566697) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481000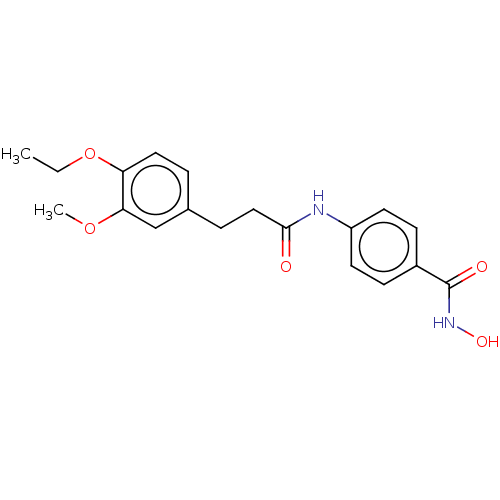 (CHEMBL568886) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308310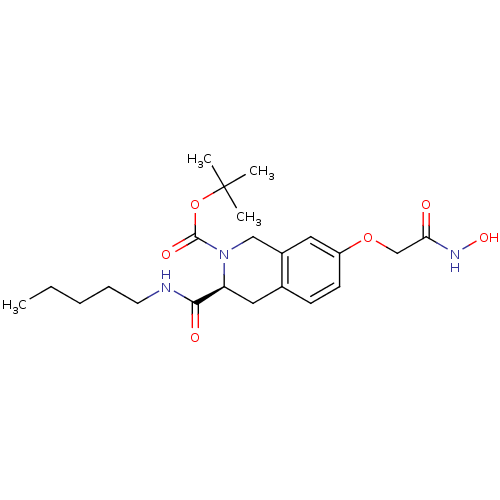 ((S)-tert-Butyl 7-(2-(hydroxyamino)-2-oxoethoxy)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308301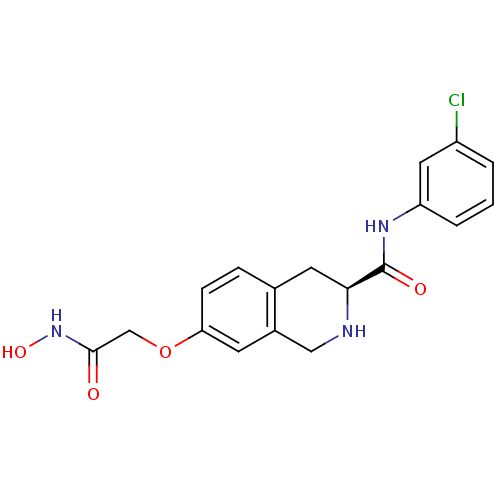 ((S)-N-(3-chlorophenyl)-7-(2-(hydroxyamino)-2-oxoet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308299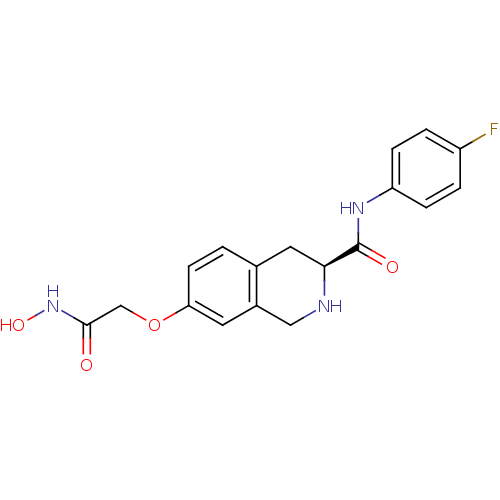 ((S)-N-(4-Fluorophenyl)-7-(2-(hydroxyamino)-2-oxoet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50480999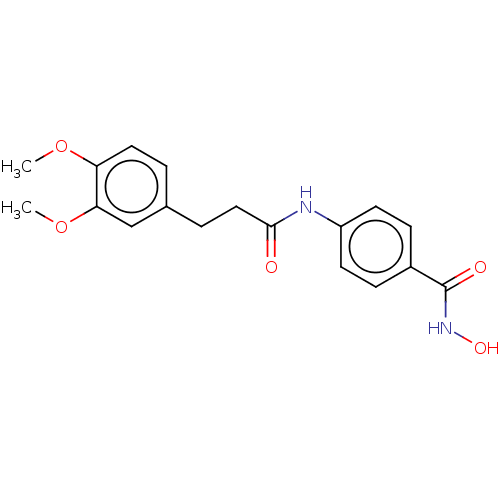 (CHEMBL570050) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308305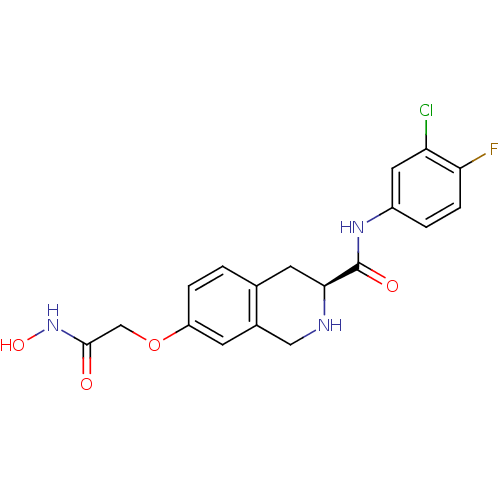 ((S)-N-(3-Chloro-4-fluorophenyl)-7-(2-(hydroxyamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308303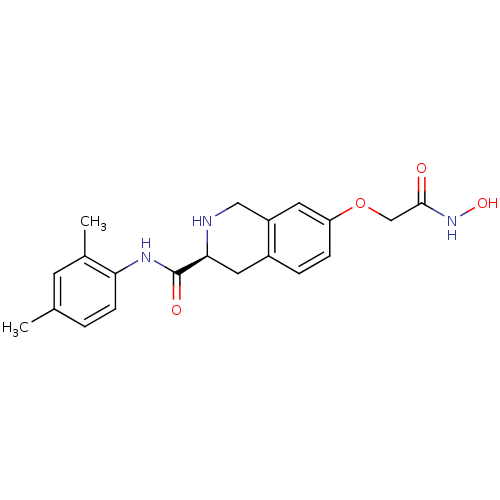 ((S)-N-(2,4-Dimethylphenyl)-7-(2-(hydroxyamino)-2-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM232987 ((E)-3-(4-(4-methoxybenzyloxy)-3-methoxyphenyl)-N-h...) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shan Dong University | Assay Description In vitro bioactivity evaluation of compounds 4a-n was performed by HDAC activity assays using a HDAC colorimetric activity assay kit (AK501; Biomol R... | J Enzyme Inhib Med Chem 25: 132-8 (2010) Article DOI: 10.3109/14756360903049034 BindingDB Entry DOI: 10.7270/Q2MS3RNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308286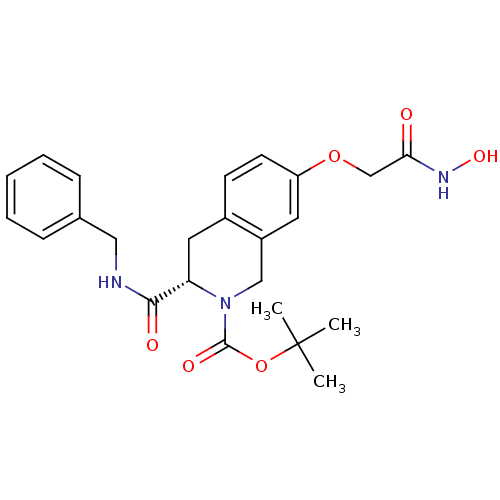 ((S)-tert-Butyl 3-(benzylcarbamoyl)-7-(2-(hydroxyam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308311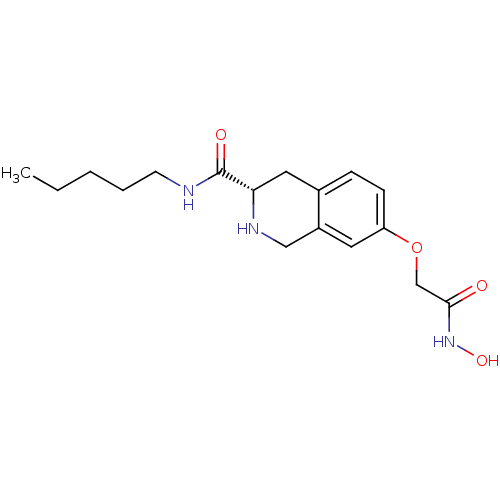 ((S)-7-(2-(Hydroxyamino)-2-oxoethoxy)-N-pentyl-1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308297 ((S)-7-(2-(Hydroxyamino)-2-oxoethoxy)-N-m-tolyl-1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308302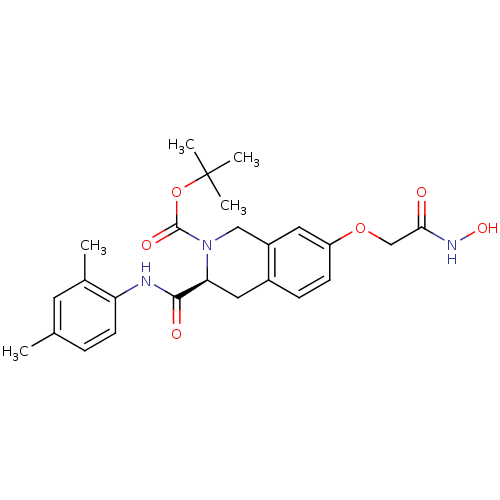 ((S)-tert-Butyl 3-(2, 4-dimethylphenylcarbamoyl)-7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308293 ((S)-7-(2-(Hydroxyamino)-2-oxoethoxy)-N-p-tolyl-1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481003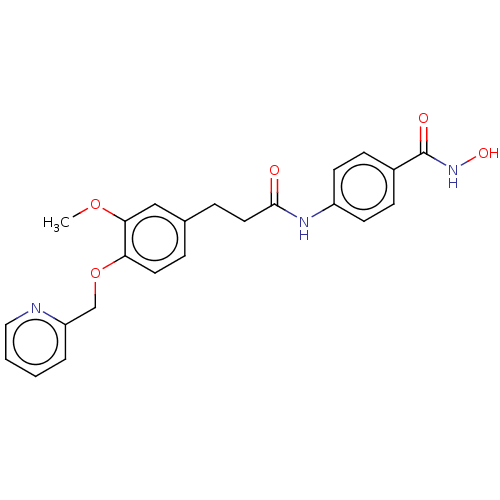 (CHEMBL567971) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308294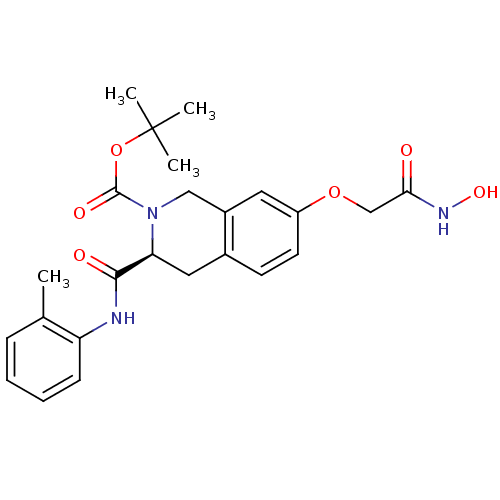 ((S)-tert-Butyl 7-(2-(hydroxyamino)-2-oxoethoxy)-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (HDAC1 and HDAC2) (Homo sapiens (Human)) | BDBM50481008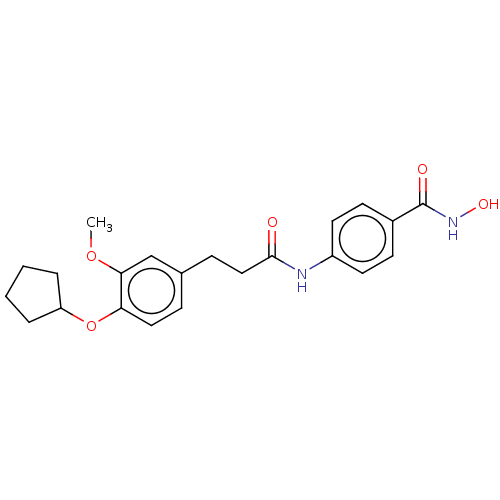 (CHEMBL583409) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC1/HDAC2 from human Hela nuclear extracts using [3H]acetylated histone peptide by colorimetry | Eur J Med Chem 44: 4470-6 (2009) Article DOI: 10.1016/j.ejmech.2009.06.010 BindingDB Entry DOI: 10.7270/Q2P55RB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308289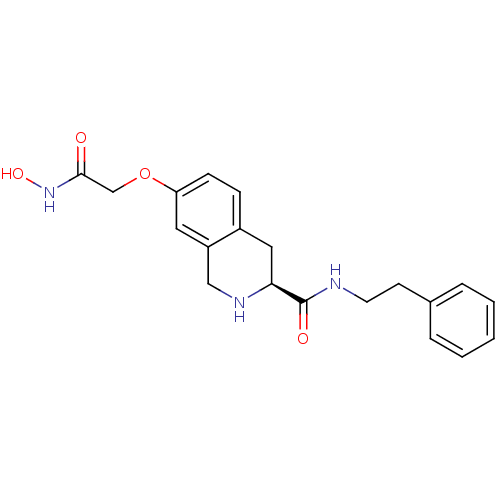 ((S)-7-(2-(Hydroxyamino)-2-oxoethoxy)-N-phenethyl-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50308295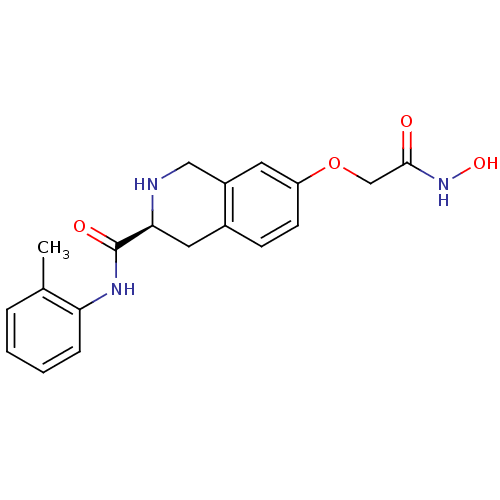 ((S)-7-(2-(Hydroxyamino)-2-oxoethoxy)-N-o-tolyl-1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in Escherichia coli assessed as Boc-Lys (acetyl)-AMC substrate hydrolysis by fluorimetric assay | Bioorg Med Chem 18: 1761-72 (2010) Article DOI: 10.1016/j.bmc.2010.01.060 BindingDB Entry DOI: 10.7270/Q2JD4WX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 73 total ) | Next | Last >> |