 Found 54 hits with Last Name = 'mahadevaswamy' and Initial = 'j'
Found 54 hits with Last Name = 'mahadevaswamy' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499993

(CHEMBL3742122)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(cc([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C16H17F3N4O4S/c1-27-7-6-21-2-4-22(5-3-21)15(24)14-20-11-8-10(16(17,18)19)9-12(23(25)26)13(11)28-14/h8-9H,2-7H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50019514

(CHEMBL3290772)Show SMILES Cn1ncc(C(=O)N2CCC(CC2)Nc2cc(=O)n(C)c3ccccc23)c1Cl Show InChI InChI=1S/C20H22ClN5O2/c1-24-17-6-4-3-5-14(17)16(11-18(24)27)23-13-7-9-26(10-8-13)20(28)15-12-22-25(2)19(15)21/h3-6,11-13,23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50019516

(CHEMBL3291063)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1n[nH]c3ccc(F)cc13)N2C(=O)c1cnn(C)c1Cl |r,TLB:21:20:2.3:7.6.8,9:7:20:2.3| Show InChI InChI=1S/C19H20ClFN6O/c1-26-17(20)15(9-22-26)19(28)27-12-3-4-13(27)8-11(7-12)23-18-14-6-10(21)2-5-16(14)24-25-18/h2,5-6,9,11-13H,3-4,7-8H2,1H3,(H2,23,24,25)/t11-,12+,13- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50019515

(CHEMBL3291062)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(=O)n(C)c3cc(F)c(F)cc13)N2C(=O)c1noc(C)c1C |r,TLB:25:24:6.7.8:2.3,9:7:24:2.3| Show InChI InChI=1S/C23H24F2N4O3/c1-11-12(2)32-27-22(11)23(31)29-14-4-5-15(29)7-13(6-14)26-19-10-21(30)28(3)20-9-18(25)17(24)8-16(19)20/h8-10,13-15,26H,4-7H2,1-3H3/t13-,14+,15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50019519
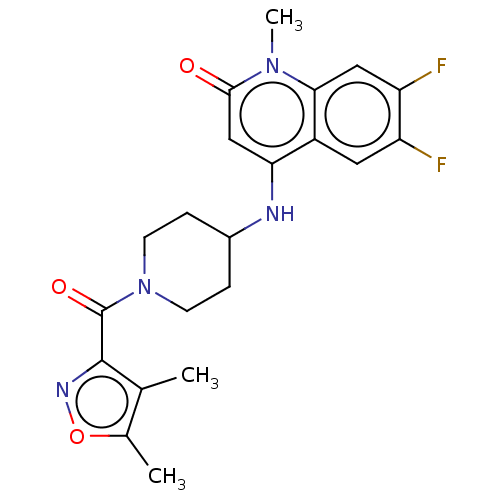
(CHEMBL3291056)Show SMILES Cc1onc(C(=O)N2CCC(CC2)Nc2cc(=O)n(C)c3cc(F)c(F)cc23)c1C Show InChI InChI=1S/C21H22F2N4O3/c1-11-12(2)30-25-20(11)21(29)27-6-4-13(5-7-27)24-17-10-19(28)26(3)18-9-16(23)15(22)8-14(17)18/h8-10,13,24H,4-7H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of adenosine A1 receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499994
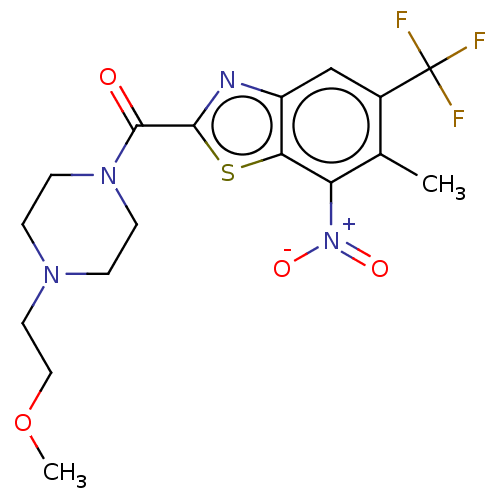
(CHEMBL3741545)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(c(C)c([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O4S/c1-10-11(17(18,19)20)9-12-14(13(10)24(26)27)29-15(21-12)16(25)23-5-3-22(4-6-23)7-8-28-2/h9H,3-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 9.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019515

(CHEMBL3291062)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(=O)n(C)c3cc(F)c(F)cc13)N2C(=O)c1noc(C)c1C |r,TLB:25:24:6.7.8:2.3,9:7:24:2.3| Show InChI InChI=1S/C23H24F2N4O3/c1-11-12(2)32-27-22(11)23(31)29-14-4-5-15(29)7-13(6-14)26-19-10-21(30)28(3)20-9-18(25)17(24)8-16(19)20/h8-10,13-15,26H,4-7H2,1-3H3/t13-,14+,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499993

(CHEMBL3742122)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(cc([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C16H17F3N4O4S/c1-27-7-6-21-2-4-22(5-3-21)15(24)14-20-11-8-10(16(17,18)19)9-12(23(25)26)13(11)28-14/h8-9H,2-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499993

(CHEMBL3742122)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(cc([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C16H17F3N4O4S/c1-27-7-6-21-2-4-22(5-3-21)15(24)14-20-11-8-10(16(17,18)19)9-12(23(25)26)13(11)28-14/h8-9H,2-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50019511

(CHEMBL3290766)Show SMILES Cn1ncc(C(=O)N2CCC(CC2)Nc2cc(=O)[nH]c3ccccc23)c1Cl Show InChI InChI=1S/C19H20ClN5O2/c1-24-18(20)14(11-21-24)19(27)25-8-6-12(7-9-25)22-16-10-17(26)23-15-5-3-2-4-13(15)16/h2-5,10-12H,6-9H2,1H3,(H2,22,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mu opioid receptor (unknown origin) |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50499995
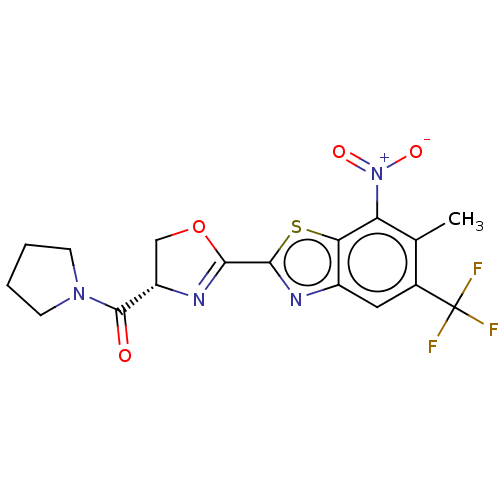
(CHEMBL3739831)Show SMILES Cc1c(cc2nc(sc2c1[N+]([O-])=O)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:15| Show InChI InChI=1S/C17H15F3N4O4S/c1-8-9(17(18,19)20)6-10-13(12(8)24(26)27)29-15(22-10)14-21-11(7-28-14)16(25)23-4-2-3-5-23/h6,11H,2-5,7H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50499991

(CHEMBL3742347)Show SMILES [O-][N+](=O)c1cc(cc2[n+]([O-])c(sc12)C(=O)N1CCCCC1)C(F)(F)F Show InChI InChI=1S/C14H12F3N3O4S/c15-14(16,17)8-6-9-11(10(7-8)20(23)24)25-13(19(9)22)12(21)18-4-2-1-3-5-18/h6-7H,1-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C9 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019654

(CHEMBL3109802)Show InChI InChI=1S/C18H21N5O3/c1-11-6-15-16(20-7-11)13(17(25)19-4-5-24)8-23(15)9-14-12(2)18(26-3)22-10-21-14/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019516

(CHEMBL3291063)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1n[nH]c3ccc(F)cc13)N2C(=O)c1cnn(C)c1Cl |r,TLB:21:20:2.3:7.6.8,9:7:20:2.3| Show InChI InChI=1S/C19H20ClFN6O/c1-26-17(20)15(9-22-26)19(28)27-12-3-4-13(27)8-11(7-12)23-18-14-6-10(21)2-5-16(14)24-25-18/h2,5-6,9,11-13H,3-4,7-8H2,1H3,(H2,23,24,25)/t11-,12+,13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50516044
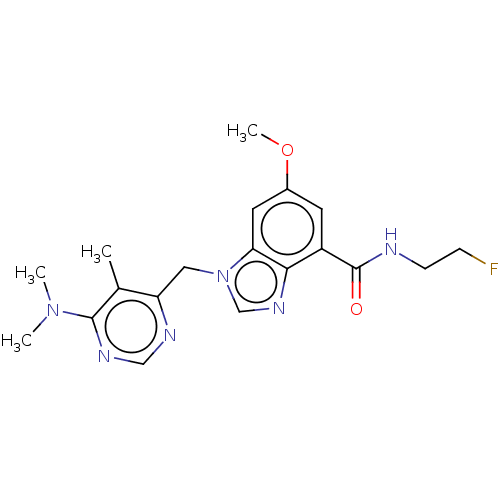
(CHEMBL4515785)Show SMILES COc1cc(C(=O)NCCF)c2ncn(Cc3ncnc(N(C)C)c3C)c2c1 Show InChI InChI=1S/C19H23FN6O2/c1-12-15(22-10-23-18(12)25(2)3)9-26-11-24-17-14(19(27)21-6-5-20)7-13(28-4)8-16(17)26/h7-8,10-11H,5-6,9H2,1-4H3,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019517

(CHEMBL3291065)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(=O)n(C)c3ncccc13)N2C(=O)c1cnn(C)c1Cl |r,TLB:23:22:2.3:7.6.8,9:7:22:2.3| Show InChI InChI=1S/C21H23ClN6O2/c1-26-18(29)10-17(15-4-3-7-23-20(15)26)25-12-8-13-5-6-14(9-12)28(13)21(30)16-11-24-27(2)19(16)22/h3-4,7,10-14,25H,5-6,8-9H2,1-2H3/t12-,13+,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50516045
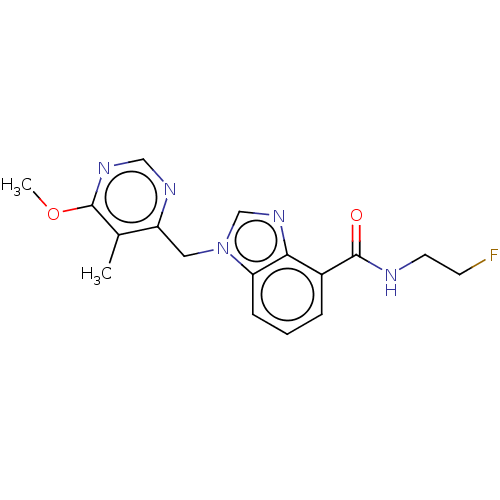
(CHEMBL4473847)Show InChI InChI=1S/C17H18FN5O2/c1-11-13(20-9-21-17(11)25-2)8-23-10-22-15-12(4-3-5-14(15)23)16(24)19-7-6-18/h3-5,9-10H,6-8H2,1-2H3,(H,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019518

(CHEMBL3291066)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(=O)n(C)c3nccnc13)N2C(=O)c1noc(C)c1C |r,TLB:23:22:6.7.8:2.3,9:7:22:2.3| Show InChI InChI=1S/C21H24N6O3/c1-11-12(2)30-25-18(11)21(29)27-14-4-5-15(27)9-13(8-14)24-16-10-17(28)26(3)20-19(16)22-6-7-23-20/h6-7,10,13-15,24H,4-5,8-9H2,1-3H3/t13-,14+,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50019514

(CHEMBL3290772)Show SMILES Cn1ncc(C(=O)N2CCC(CC2)Nc2cc(=O)n(C)c3ccccc23)c1Cl Show InChI InChI=1S/C20H22ClN5O2/c1-24-17-6-4-3-5-14(17)16(11-18(24)27)23-13-7-9-26(10-8-13)20(28)15-12-22-25(2)19(15)21/h3-6,11-13,23H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by medium-throughput electrophysiology |
J Med Chem 57: 5419-34 (2014)
Article DOI: 10.1021/jm5005978
BindingDB Entry DOI: 10.7270/Q25Q4XNM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50019654

(CHEMBL3109802)Show InChI InChI=1S/C18H21N5O3/c1-11-6-15-16(20-7-11)13(17(25)19-4-5-24)8-23(15)9-14-12(2)18(26-3)22-10-21-14/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50516045

(CHEMBL4473847)Show InChI InChI=1S/C17H18FN5O2/c1-11-13(20-9-21-17(11)25-2)8-23-10-22-15-12(4-3-5-14(15)23)16(24)19-7-6-18/h3-5,9-10H,6-8H2,1-2H3,(H,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50516044

(CHEMBL4515785)Show SMILES COc1cc(C(=O)NCCF)c2ncn(Cc3ncnc(N(C)C)c3C)c2c1 Show InChI InChI=1S/C19H23FN6O2/c1-12-15(22-10-23-18(12)25(2)3)9-26-11-24-17-14(19(27)21-6-5-20)7-13(28-4)8-16(17)26/h7-8,10-11H,5-6,9H2,1-4H3,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50516045

(CHEMBL4473847)Show InChI InChI=1S/C17H18FN5O2/c1-11-13(20-9-21-17(11)25-2)8-23-10-22-15-12(4-3-5-14(15)23)16(24)19-7-6-18/h3-5,9-10H,6-8H2,1-2H3,(H,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50516045

(CHEMBL4473847)Show InChI InChI=1S/C17H18FN5O2/c1-11-13(20-9-21-17(11)25-2)8-23-10-22-15-12(4-3-5-14(15)23)16(24)19-7-6-18/h3-5,9-10H,6-8H2,1-2H3,(H,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50516045

(CHEMBL4473847)Show InChI InChI=1S/C17H18FN5O2/c1-11-13(20-9-21-17(11)25-2)8-23-10-22-15-12(4-3-5-14(15)23)16(24)19-7-6-18/h3-5,9-10H,6-8H2,1-2H3,(H,19,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50516044

(CHEMBL4515785)Show SMILES COc1cc(C(=O)NCCF)c2ncn(Cc3ncnc(N(C)C)c3C)c2c1 Show InChI InChI=1S/C19H23FN6O2/c1-12-15(22-10-23-18(12)25(2)3)9-26-11-24-17-14(19(27)21-6-5-20)7-13(28-4)8-16(17)26/h7-8,10-11H,5-6,9H2,1-4H3,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50019654

(CHEMBL3109802)Show InChI InChI=1S/C18H21N5O3/c1-11-6-15-16(20-7-11)13(17(25)19-4-5-24)8-23(15)9-14-12(2)18(26-3)22-10-21-14/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50516044

(CHEMBL4515785)Show SMILES COc1cc(C(=O)NCCF)c2ncn(Cc3ncnc(N(C)C)c3C)c2c1 Show InChI InChI=1S/C19H23FN6O2/c1-12-15(22-10-23-18(12)25(2)3)9-26-11-24-17-14(19(27)21-6-5-20)7-13(28-4)8-16(17)26/h7-8,10-11H,5-6,9H2,1-4H3,(H,21,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50516044

(CHEMBL4515785)Show SMILES COc1cc(C(=O)NCCF)c2ncn(Cc3ncnc(N(C)C)c3C)c2c1 Show InChI InChI=1S/C19H23FN6O2/c1-12-15(22-10-23-18(12)25(2)3)9-26-11-24-17-14(19(27)21-6-5-20)7-13(28-4)8-16(17)26/h7-8,10-11H,5-6,9H2,1-4H3,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50516045

(CHEMBL4473847)Show InChI InChI=1S/C17H18FN5O2/c1-11-13(20-9-21-17(11)25-2)8-23-10-22-15-12(4-3-5-14(15)23)16(24)19-7-6-18/h3-5,9-10H,6-8H2,1-2H3,(H,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50019654

(CHEMBL3109802)Show InChI InChI=1S/C18H21N5O3/c1-11-6-15-16(20-7-11)13(17(25)19-4-5-24)8-23(15)9-14-12(2)18(26-3)22-10-21-14/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50516044

(CHEMBL4515785)Show SMILES COc1cc(C(=O)NCCF)c2ncn(Cc3ncnc(N(C)C)c3C)c2c1 Show InChI InChI=1S/C19H23FN6O2/c1-12-15(22-10-23-18(12)25(2)3)9-26-11-24-17-14(19(27)21-6-5-20)7-13(28-4)8-16(17)26/h7-8,10-11H,5-6,9H2,1-4H3,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50019654

(CHEMBL3109802)Show InChI InChI=1S/C18H21N5O3/c1-11-6-15-16(20-7-11)13(17(25)19-4-5-24)8-23(15)9-14-12(2)18(26-3)22-10-21-14/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50019654

(CHEMBL3109802)Show InChI InChI=1S/C18H21N5O3/c1-11-6-15-16(20-7-11)13(17(25)19-4-5-24)8-23(15)9-14-12(2)18(26-3)22-10-21-14/h6-8,10,24H,4-5,9H2,1-3H3,(H,19,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
ACS Med Chem Lett 10: 1480-1485 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00343
BindingDB Entry DOI: 10.7270/Q2T43XFK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499992

(CHEMBL3739863)Show SMILES [O-][N+](=O)c1cc(cc2nc(sc12)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:14| Show InChI InChI=1S/C16H13F3N4O4S/c17-16(18,19)8-5-9-12(11(6-8)23(25)26)28-14(21-9)13-20-10(7-27-13)15(24)22-3-1-2-4-22/h5-6,10H,1-4,7H2/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50499993

(CHEMBL3742122)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(cc([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C16H17F3N4O4S/c1-27-7-6-21-2-4-22(5-3-21)15(24)14-20-11-8-10(16(17,18)19)9-12(23(25)26)13(11)28-14/h8-9H,2-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50499994

(CHEMBL3741545)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(c(C)c([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O4S/c1-10-11(17(18,19)20)9-12-14(13(10)24(26)27)29-15(21-12)16(25)23-5-3-22(4-6-23)7-8-28-2/h9H,3-8H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2D6 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50499994

(CHEMBL3741545)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(c(C)c([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O4S/c1-10-11(17(18,19)20)9-12-14(13(10)24(26)27)29-15(21-12)16(25)23-5-3-22(4-6-23)7-8-28-2/h9H,3-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50499995

(CHEMBL3739831)Show SMILES Cc1c(cc2nc(sc2c1[N+]([O-])=O)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:15| Show InChI InChI=1S/C17H15F3N4O4S/c1-8-9(17(18,19)20)6-10-13(12(8)24(26)27)29-15(22-10)14-21-11(7-28-14)16(25)23-4-2-3-5-23/h6,11H,2-5,7H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499994

(CHEMBL3741545)Show SMILES COCCN1CCN(CC1)C(=O)c1nc2cc(c(C)c([N+]([O-])=O)c2s1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O4S/c1-10-11(17(18,19)20)9-12-14(13(10)24(26)27)29-15(21-12)16(25)23-5-3-22(4-6-23)7-8-28-2/h9H,3-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50499995

(CHEMBL3739831)Show SMILES Cc1c(cc2nc(sc2c1[N+]([O-])=O)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:15| Show InChI InChI=1S/C17H15F3N4O4S/c1-8-9(17(18,19)20)6-10-13(12(8)24(26)27)29-15(22-10)14-21-11(7-28-14)16(25)23-4-2-3-5-23/h6,11H,2-5,7H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50499995

(CHEMBL3739831)Show SMILES Cc1c(cc2nc(sc2c1[N+]([O-])=O)C1=N[C@@H](CO1)C(=O)N1CCCC1)C(F)(F)F |r,t:15| Show InChI InChI=1S/C17H15F3N4O4S/c1-8-9(17(18,19)20)6-10-13(12(8)24(26)27)29-15(22-10)14-21-11(7-28-14)16(25)23-4-2-3-5-23/h6,11H,2-5,7H2,1H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 by fluorescence assay |
Bioorg Med Chem 23: 7694-710 (2015)
Article DOI: 10.1016/j.bmc.2015.11.017
BindingDB Entry DOI: 10.7270/Q2PR800F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data