

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RNA-directed RNA polymerase (Hepacivirus C) | BDBM50484982 (CHEMBL2017865) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant | Bioorg Med Chem Lett 22: 2866-71 (2012) Article DOI: 10.1016/j.bmcl.2012.02.063 BindingDB Entry DOI: 10.7270/Q2NK3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepacivirus C) | BDBM50484986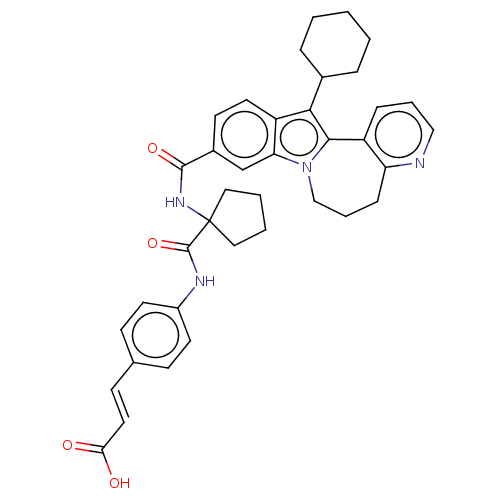 (CHEMBL2017863) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant | Bioorg Med Chem Lett 22: 2866-71 (2012) Article DOI: 10.1016/j.bmcl.2012.02.063 BindingDB Entry DOI: 10.7270/Q2NK3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepacivirus C) | BDBM50484981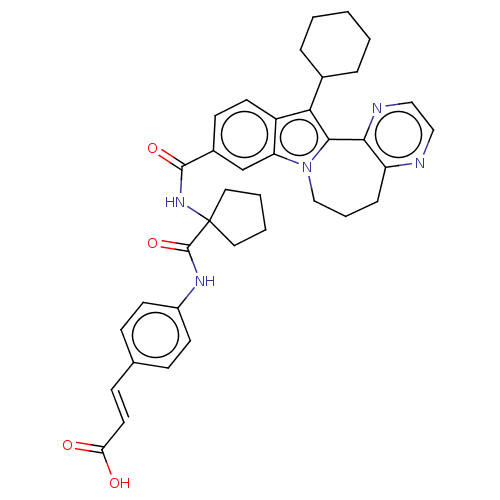 (CHEMBL2017862) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant | Bioorg Med Chem Lett 22: 2866-71 (2012) Article DOI: 10.1016/j.bmcl.2012.02.063 BindingDB Entry DOI: 10.7270/Q2NK3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepacivirus C) | BDBM50484983 (CHEMBL2017866) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant | Bioorg Med Chem Lett 22: 2866-71 (2012) Article DOI: 10.1016/j.bmcl.2012.02.063 BindingDB Entry DOI: 10.7270/Q2NK3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepacivirus C) | BDBM50484985 (CHEMBL2017864) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant | Bioorg Med Chem Lett 22: 2866-71 (2012) Article DOI: 10.1016/j.bmcl.2012.02.063 BindingDB Entry DOI: 10.7270/Q2NK3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepacivirus C) | BDBM50484977 (CHEMBL2017856) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant | Bioorg Med Chem Lett 22: 2866-71 (2012) Article DOI: 10.1016/j.bmcl.2012.02.063 BindingDB Entry DOI: 10.7270/Q2NK3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepacivirus C) | BDBM50484984 (CHEMBL2017858) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant | Bioorg Med Chem Lett 22: 2866-71 (2012) Article DOI: 10.1016/j.bmcl.2012.02.063 BindingDB Entry DOI: 10.7270/Q2NK3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepacivirus C) | BDBM50484980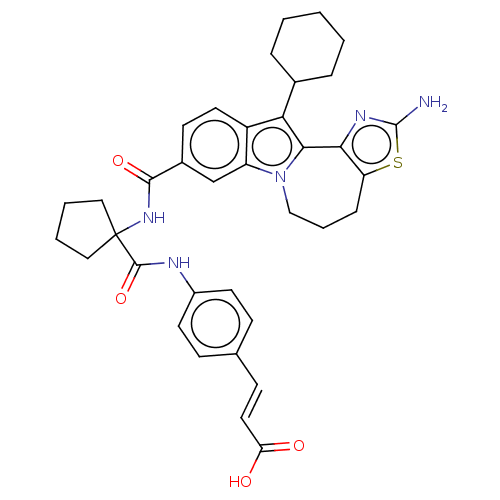 (CHEMBL2017861) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant | Bioorg Med Chem Lett 22: 2866-71 (2012) Article DOI: 10.1016/j.bmcl.2012.02.063 BindingDB Entry DOI: 10.7270/Q2NK3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepacivirus C) | BDBM50484979 (CHEMBL2017860) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant | Bioorg Med Chem Lett 22: 2866-71 (2012) Article DOI: 10.1016/j.bmcl.2012.02.063 BindingDB Entry DOI: 10.7270/Q2NK3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepacivirus C) | BDBM50484978 (CHEMBL2017857) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant | Bioorg Med Chem Lett 22: 2866-71 (2012) Article DOI: 10.1016/j.bmcl.2012.02.063 BindingDB Entry DOI: 10.7270/Q2NK3HWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50496099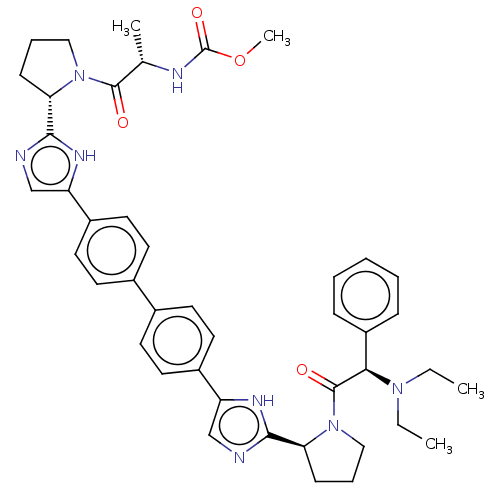 (CHEMBL3121151) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG by whole cell patch clamp assay | J Med Chem 57: 2013-32 (2014) Article DOI: 10.1021/jm401836p BindingDB Entry DOI: 10.7270/Q28918V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50142902 (CHEMBL3760108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG by FLIPR assay | Bioorg Med Chem Lett 26: 936-40 (2016) Article DOI: 10.1016/j.bmcl.2015.12.058 BindingDB Entry DOI: 10.7270/Q2ZG6V33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase beta (Homo sapiens (Human)) | BDBM50268397 (CHEMBL4067852) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase beta | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase alpha catalytic subunit (Homo sapiens (Human)) | BDBM50268397 (CHEMBL4067852) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Inhibition of human DNA polymerase alpha | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50448498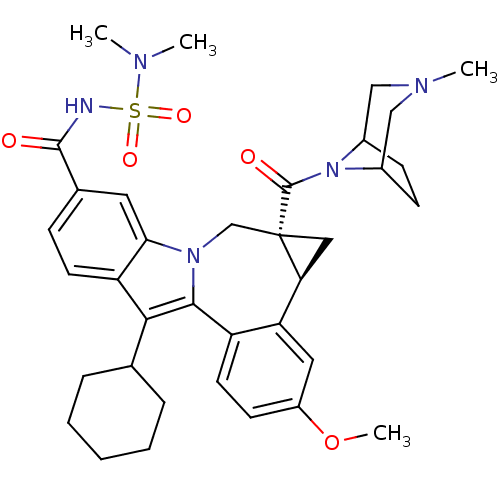 (BMS-791325 | Beclabuvir) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG by FLIPR assay | Bioorg Med Chem Lett 26: 936-40 (2016) Article DOI: 10.1016/j.bmcl.2015.12.058 BindingDB Entry DOI: 10.7270/Q2ZG6V33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50387084 (BMS-790052 | DACLATASVIR) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG by whole cell patch clamp assay | J Med Chem 57: 2013-32 (2014) Article DOI: 10.1021/jm401836p BindingDB Entry DOI: 10.7270/Q28918V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50142900 (CHEMBL3759768) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG by FLIPR assay | Bioorg Med Chem Lett 26: 936-40 (2016) Article DOI: 10.1016/j.bmcl.2015.12.058 BindingDB Entry DOI: 10.7270/Q2ZG6V33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50142901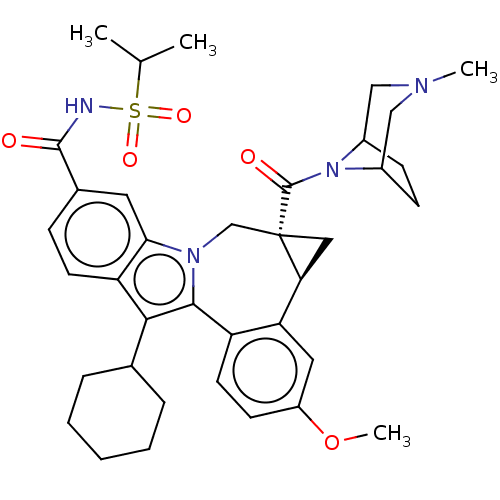 (CHEMBL3758288) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human ERG by FLIPR assay | Bioorg Med Chem Lett 26: 936-40 (2016) Article DOI: 10.1016/j.bmcl.2015.12.058 BindingDB Entry DOI: 10.7270/Q2ZG6V33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50268398 (CHEMBL4061940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50142901 (CHEMBL3758288) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50268399 (CHEMBL4094467) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50268400 (CHEMBL4081189) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.33E+3 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50268401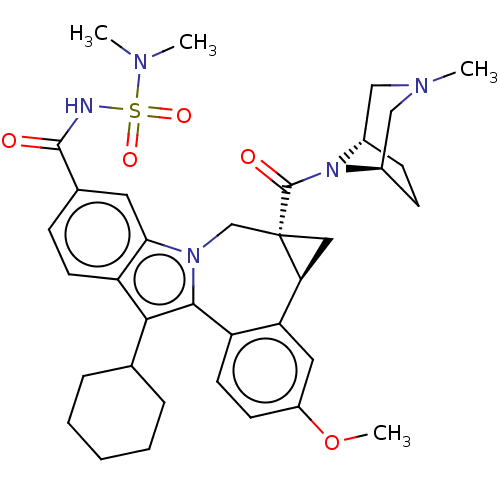 (CHEMBL4105584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50268397 (CHEMBL4067852) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng Curated by ChEMBL | Assay Description Activation of PXR in human hepatocytes assessed as induction of CYP450 expression | Bioorg Med Chem Lett 27: 3294-3300 (2017) Article DOI: 10.1016/j.bmcl.2017.06.024 BindingDB Entry DOI: 10.7270/Q20004KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||