

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1727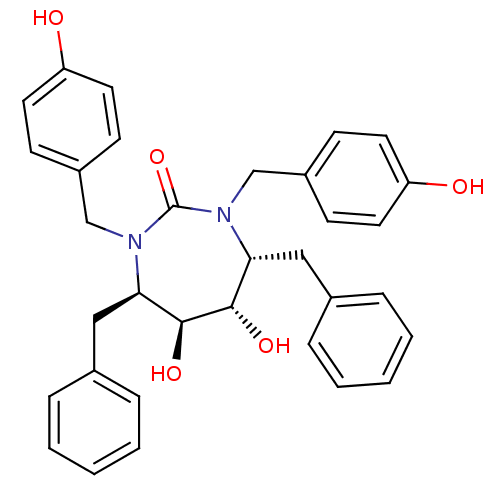 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1726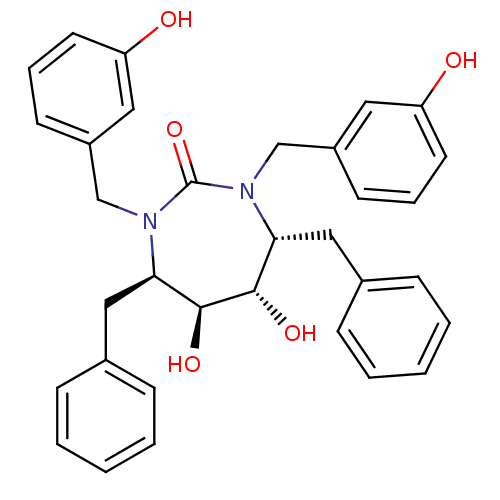 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1730 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1702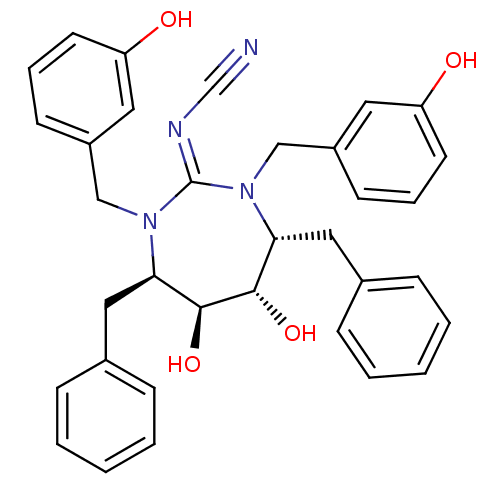 (Cyclic Cyanoguanidine deriv. 8r | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.720 | -54.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1723 ((4R,5S,6S,7R)-1,3-bis[(4-aminophenyl)methyl]-4,7-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1693 (Cyclic Cyanoguanidine deriv. 8i | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | -52.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1706 (Cyclic Cyanoguanidine deriv. 8v | [4R-(4a,5a,6b,7b...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | -52.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1692 (Cyclic Cyanoguanidine deriv. 8h | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -51.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1703 (Cyclic Cyanoguanidine deriv. 8s | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60 | -51.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1688 (Cyclic Cyanoguanidine deriv. 8d | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70 | -50.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1720 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1724 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-3-[(3-cyanophenyl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1690 (Cyclic Cyanoguanidine deriv. 8f | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.80 | -50.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1694 (Cyclic Cyanoguanidine deriv. 8j | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.70 | -48.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1698 (Cyclic Cyanoguanidine deriv. 8n | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.40 | -48.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1707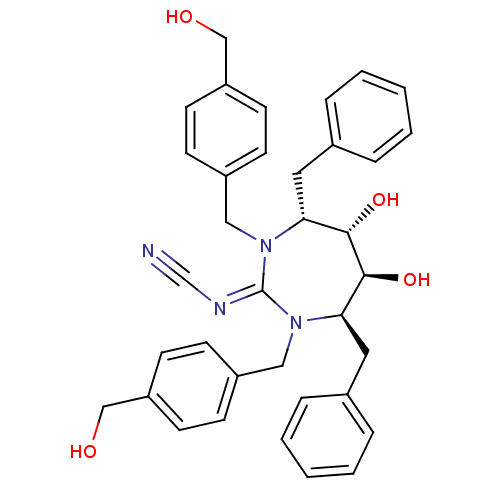 (Cyclic Cyanoguanidine deriv. 8w | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 11 | -47.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1687 (Cyclic Cyanoguanidine deriv. 8c | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -46.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1695 (Cyclic Cyanoguanidine deriv. 8k | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1691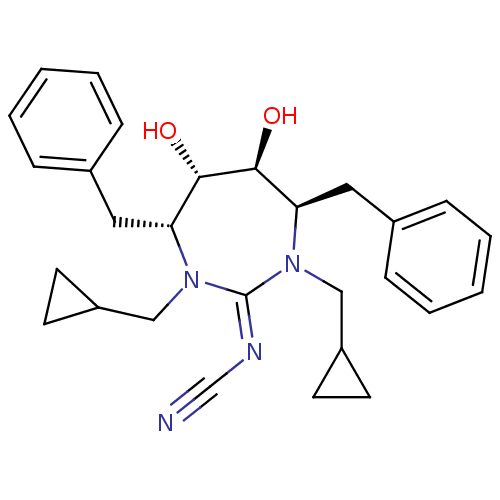 (Cyclic Cyanoguanidine deriv. 8g | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | -45.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1708 (Cyclic Cyanoguanidine deriv. 8x | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | -45.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1699 (Cyclic Cyanoguanidine deriv. 8o | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | -45.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1700 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-2-(cyanoimino)-3-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 27 | -44.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1689 (Cyclic Cyanoguanidine deriv. 8e | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -44.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1721 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1686 (Cyclic Cyanoguanidine deriv. 8b | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 37 | -44.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1725 (4-{[(4R,5S,6S,7R)-4,7-dibenzyl-3-[(4-cyanophenyl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1697 (Cyclic Cyanoguanidine deriv. 8m | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 67 | -42.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1696 (Cyclic Cyanoguanidine deriv. 8l | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 89 | -41.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1701 (4-{[(4R,5S,6S,7R)-4,7-dibenzyl-2-(cyanoimino)-3-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 128 | -40.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1709 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-diaze...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1728 ((4R,5S,6S,7R)-4,7-dibenzyl-1,3-bis({[3-(benzyloxy)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1729 ((4R,5S,6S,7R)-4,7-dibenzyl-1,3-bis({[4-(benzyloxy)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 542 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1705 (Cyclic Cyanoguanidine deriv. 8u | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 900 | -35.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1704 (Cyclic Cyanoguanidine deriv. 8t | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.37E+3 | -34.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1685 (Cyclic Cyanoguanidine deriv. 8a | [(4R,5S,6S,7R)-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.25E+4 | >-29.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50226528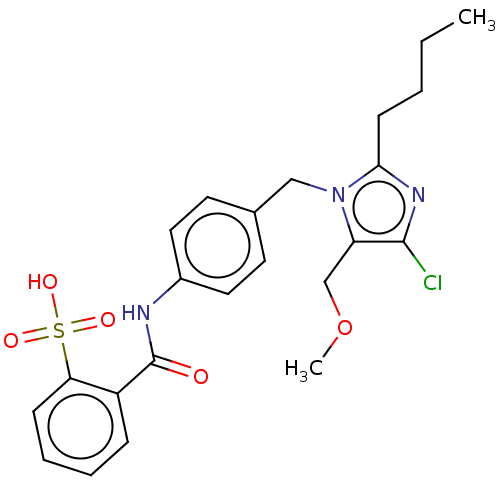 (CHEMBL284425) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50226522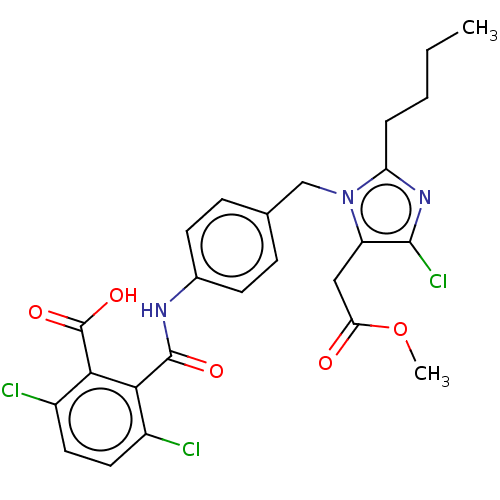 (CHEMBL1744348) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50226421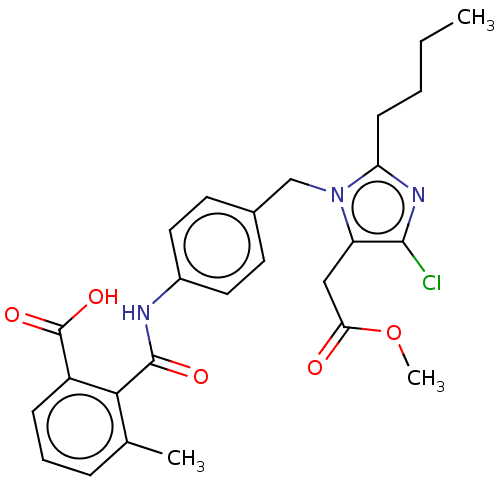 (CHEMBL281589) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50226467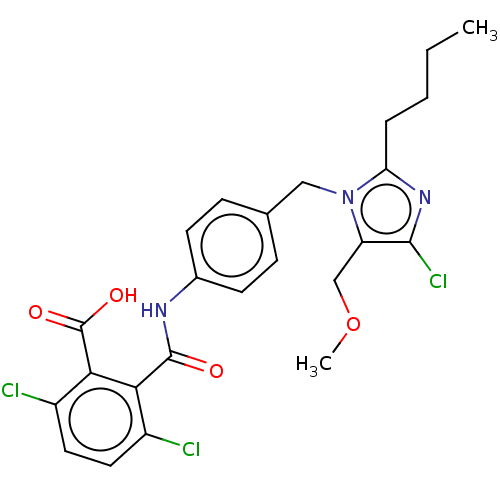 (CHEMBL276956) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50087583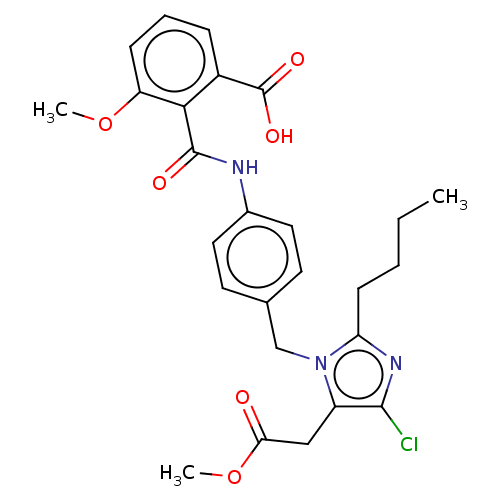 (CHEMBL25031) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50015270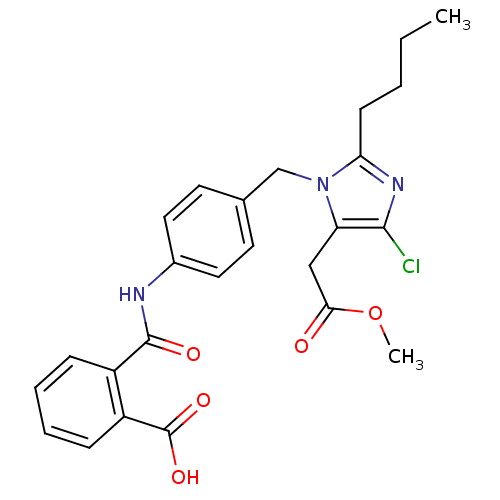 (CHEMBL262958 | N-[4-(2-Butyl-4-chloro-5-methoxycar...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50226428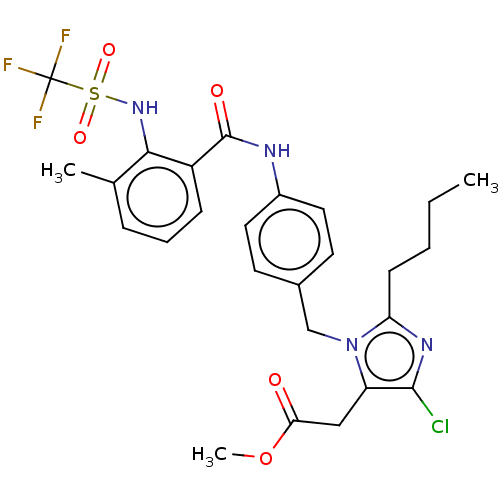 (CHEMBL28011) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50226532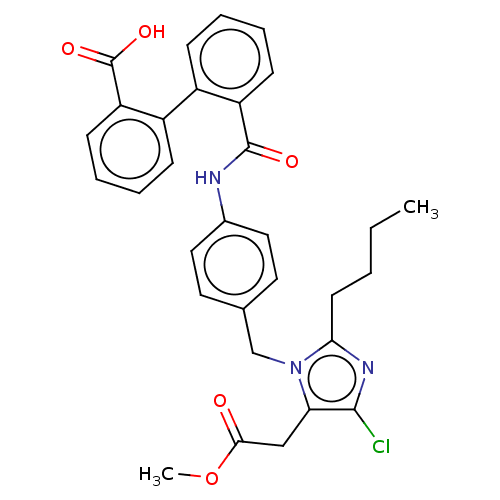 (CHEMBL282491) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50226525 (CHEMBL26652) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin ll receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50023646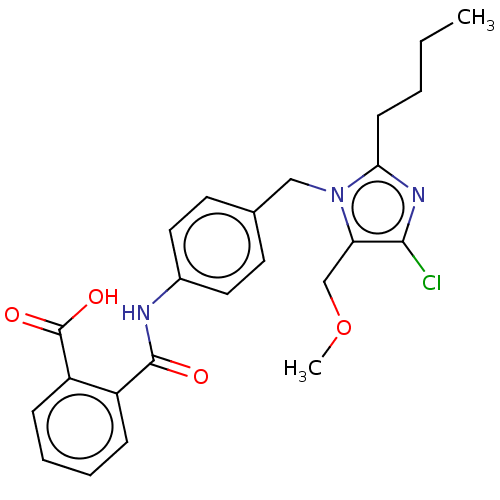 (CHEMBL24364) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50087614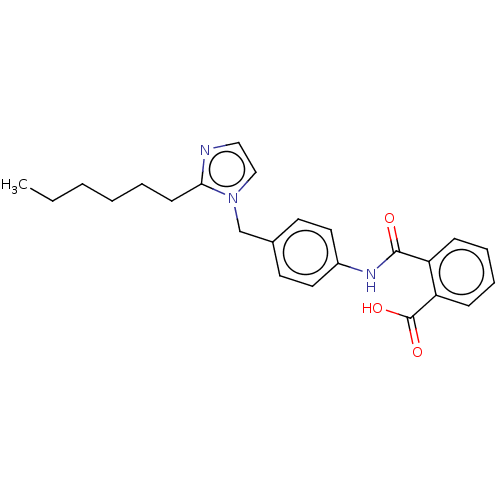 (CHEMBL27981) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin ll receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50226523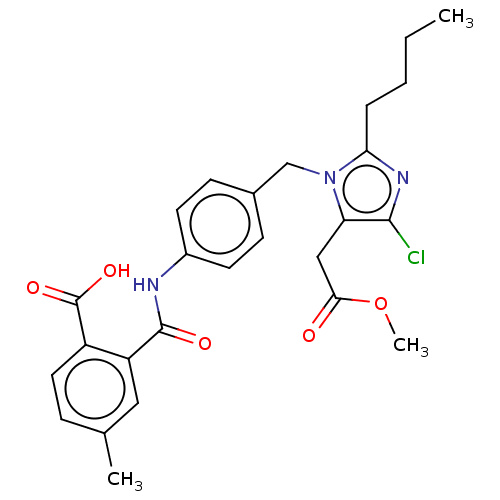 (CHEMBL283071) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50108642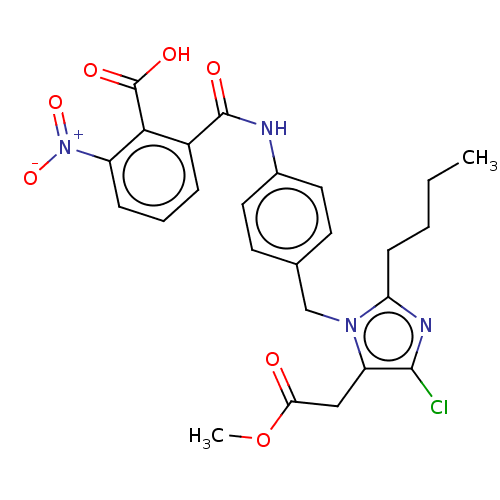 (CHEMBL27725) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50222263 (CHEMBL27839) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50226425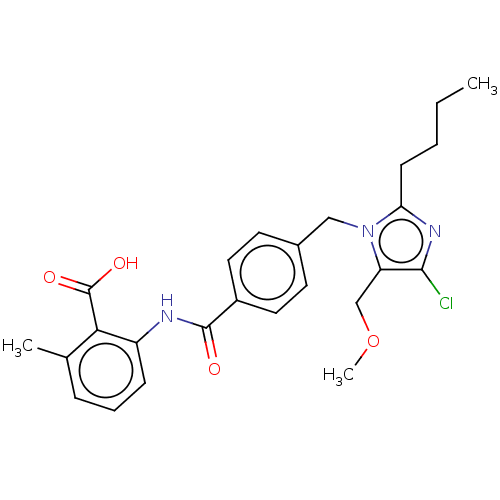 (CHEMBL28391) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
E. I. du Pont de Nemours& Company, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration that gave 50 percent displacement of specific binding of [3H]AII (2 nM) to Angiotensin II receptor | J Med Chem 33: 1312-29 (1990) BindingDB Entry DOI: 10.7270/Q2TM7DCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 116 total ) | Next | Last >> |